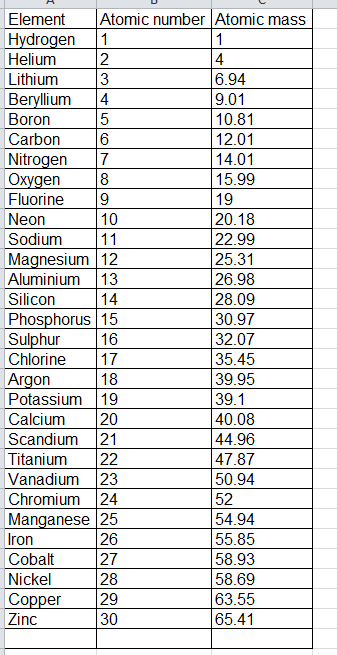
1 to 30 elements atomic mass
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest.
The atomic mass of an element is the average mass of its atoms measured in atomic mass units amu, commonly known as daltons, D. In this article, we will learn about the Atomic Mass of Elements 1 to 30 with Symbols. The absolute mass of a single atom is its atomic mass, which is measured in atomic mass units, or amu. Carbon, for example, is a typical carbon atom with six neutrons and six protons. It has a mass of 12 atomic units. In most cases, the atomic mass number is rounded to the next whole number.
1 to 30 elements atomic mass
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. Since we have seen the definition of atomic mass let us discuss it in detail. The atomic mass of a solitary atom is its absolute mass and is regularly expressed in atomic mass units or amu. For example, a normal carbon atom with six neutrons and six protons is denoted as carbon It has an atomic mass equal to 12 amu. The atomic mass number is usually rounded off to the nearest whole number. Since an element's isotopes have distinctive atomic masses, researchers may likewise decide the general atomic mass—once in a while called the atomic weight—for an element. The general atomic mass is the normal of the atomic masses of the apparent multitude of various isotopes in an example. Every isotope's contribution to the normal is controlled by how huge a fraction of the example it makes up.
Atomic mass cannot be used for defining the type of element. For example, iron has an atomic mass of The same concept is also used to determine the molar quantities of ionic molecules and compounds.
Buka menu navigasi. Tutup saran Cari Cari. Pengaturan Pengguna. Lewati carousel. Karusel Sebelumnya. Karusel Berikutnya.
Even though atoms are very tiny pieces of matter, they have mass. Their masses are so small, however, that chemists often use a unit other than grams to express them—the atomic mass unit. Masses of other atoms are expressed with respect to the atomic mass unit. For example, the mass of an atom of 1 H is 1. Note, however, that these masses are for particular isotopes of each element.
1 to 30 elements atomic mass
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.
Target dinnerware sets
Download Now. What is the Atomic Mass of Fluorine? Message Message. What is the Significance of the Formula of a Substance? The atomic mass of oxygen is approximately The atomic mass of elements is measured with the help of unified atomic mass units. Admission Experiences. Types Of Minerals. What is the Atomic Mass of Potassium? Buka menu navigasi. What is the Atomic Mass of Lithium? This means that one atom has an atomic mass of exactly For example, the elements in Group 1A are mostly soft metals that are highly reactive with water. The atomic mass of an atom is a weighted average of the masses of the isotopes of that atom.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight.
The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. Article Tags :. Find important questions, their answers and explanations too. Elements List Elements List Profesional Dokumen. Like Article. Get paid for your published articles and stand a chance to win tablet, smartwatch and exclusive GfG goodies! For example, one mole of sodium chloride NaCl has a molecular mass of Functional Glass Coatings: George E. Tandai sebagai konten tidak pantas.

0 thoughts on “1 to 30 elements atomic mass”