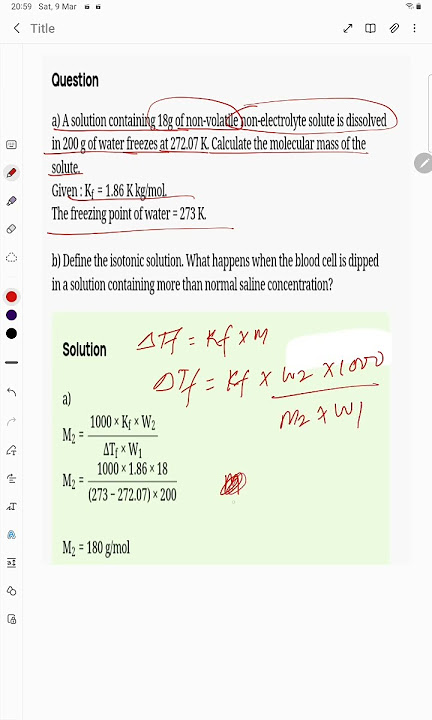
A solution containing 18g of non volatile
Posted by Maphisabet Jeengaphs 3 years, 7 months ago.
Write equations for the steps in S N 1 mechanism of the conversion of tert. Describe the three steps involved in the leaching of bauxite to get pure alumina equations not expected. An element having atomic mass A solution of 1. A solution containing 6. Mass of solute is.
A solution containing 18g of non volatile
A solution containing 18 g of anon-volitile solute in g of waer freezes at Calculate the molecular mass of the solute. State Raoult's law. Why is the vapour pressure of a solvent lowered by the addition of nonvolatile solute to it? A solution containing 18 g of non-volatile solute in g of water freezes at A solution containing 18 g of a non - electrolytic solute in g of H 2 O freezes at Find the molecular mass of the solute. A solution of 1. Calculate the molecular mass of the solute, K f for water is 1. A solution containing A solution of 7 g of a non-volatile solute in g of water boils at Find the molecular mass of th solute K b for water is The freezing point of a solution containing 18 g of a non-volatile solute dissolved in g of water decreases by 0.
Calculate the freezing point of a solution that contains 30 g urea in g water.
.
Q: An aqueous solution is 0. What is the mole fraction of each component in the…. Q: The vapor pressure of a pure volatile liquid is If NaCl s is dissolved into this liquid,…. A: The vapor pressure of a pure volatile liquid is If NaCl s is dissolved into this…. Q: The normal boiling point of diethyl ether is
A solution containing 18g of non volatile
Submitted by Elizabeth D. We will assign your question to a Numerade educator to answer. A solution containing 18g of a non-volatile non-electrolyte solute is dissolved in g of water and freezes at K.
Stan lizzie mcguire death
The freezing point of a solution containing 18 g of a non-volatile solute dissolved in g of water decreases by 0. The freezing point of a solution containing 18 g of a non-volatile solute dissolved in g of water decreases by 0. Posted by Richa Reji 2 weeks ago. What happens when the blood cell is dipped i A solution containing 18 g of …. Was this answer helpful? K f for water is 1. Posted by Shivam Yadav 1 week, 3 days ago. If K f for water is 1. View Solution.
Further, of water is then added to the solution and the new vapour pressure becomes at.
View Solution. Avoid inappropriate language and attention, vulgar terms and anything sexually suggestive. Posted by Nikita Bhatt 3 weeks, 4 days ago. Related Questions. Find the molecular mass of the solute. A solution containing 18 g of anon-volitile solute in g of waer fr Urea is a non-volatile, non-electrolytic solid. Text Solution. Once content is flagged, it is hidden from users and is reviewed by myCBSEguide team against our Community Guidelines. Gaurav Seth 3 years, 7 months ago. The freezing point of a solution containing 18 g of a non-volatile solute dissolved in g of water decreases by 0. Calculate the freezing point of a solution that contains 30 g urea in g water. When 36g of a non-volatile, non-electrolytic solute having the empirical formula C H 2 O is dissolved in 1. Question comments have also been disabled. Posted by Sankalp Yadav 1 month ago.

0 thoughts on “A solution containing 18g of non volatile”