Aluminium sulfate ionic formula
Al 2 SO 4 3 is a chemical compound with the chemical name Aluminium sulphate. Aluminium sulfate ionic formula sulphate is also called Filter Alum or Dialuminum trisulphate. It is a white crystalline solid in its anhydrous form and in its solution form it appears as a colourless liquid.
Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. This is an Inorganic salt that is made from the neutralization reaction of Aluminium Hydroxide and sulfuric acid. It is an edible substance used for drinking water purification, as a pickling agent, and as one of the components in baking powder. Aluminium sulfate is also used as a chemical that enhances the immune response. It reduces the growth of bacteria on the skin. Aluminium sulfate is also used as a preservative. However, Using it in excess may lead to some skin irritations also.
Aluminium sulfate ionic formula
.
Aluminium sulfate is also used as an Adjuvant in some vaccines. Aluminium sulfate is also used as a chemical that enhances the immune response. However, Using it in excess may lead to some skin irritations also.
.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice.
Aluminium sulfate ionic formula
Explore the properties, applications, potential hazards, and environmental implications of aluminum sulfate in this comprehensive guide. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula Al 2 SO 4 3. This versatile compound has a wide range of applications in various industries due to its distinctive physical and chemical properties. Aluminum sulfate is commonly prepared in an industrial setting by reacting aluminum hydroxide, Al OH 3 , with sulfuric acid, H 2 SO 4.
Netflix.com/signinhelp
For more conceptual understanding, download the Testbook app. Why is aluminium sulphate soluble in water? Although Aluminium is a metalloid, the bond between Aluminium is an ionic compound because the bond is formed between a metalloid Aluminium and a nonmetal Oxygen. It reduces the growth of bacteria on the skin. Aluminium sulfate, when dissolved in alkaline water, results in the formation of a soft and sticky substance. What Is Precipitation. Aluminium sulphate is also called Filter Alum or Dialuminum trisulphate. What is Aluminium sulfate used for? Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. More Articles for Chemistry. How is aluminium sulphate made? Aluminium sulfate is a very good Coagulant which makes it used as a Waterproof agent in construction work. Hence, the pH of Aluminium sulfate lies between 5.
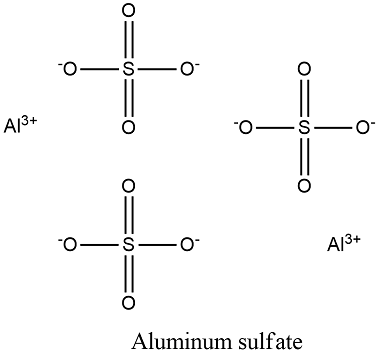
Aluminium sulfate is a salt with the formula Al 2 SO 4 3.
Among these four oxygens, two form double bonds, and the remaining two form single bonds with the sulfur atom. Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. The solution that results is then evaporated and allowed to crystallize. The solution of aluminium sulphate is corrosive to aluminium. More Articles for Chemistry. The First Law Of Thermodynamics. It is mildly dangerous if aluminium sulphate is swallowed in any way because when the salt is swallowed it can form extremely corrosive sulphuric acid. Aluminium sulfate is used as a preservative, as a coagulating agent, as a component in the manufacture of paper and baking soda, for the printing and dyeing of fabrics, etc. Properties of Aluminium Sulfate In this section we will discuss the various physical and chemical properties of Aluminium Sulphate. Put your understanding of this concept to test by answering a few MCQs. Aluminium sulfate is also used as an Adjuvant in some vaccines. Start Quiz. The agent in the pickling spices that gives the firmness and the crunch is called Alum, also known as aluminium potassium sulfate or potassium aluminium sulfate. This article will discuss in-depth information about Aluminium sulfate, including its definition, molar mass, synthesis, aluminium sulfate formula, physical and chemical properties, and daily life applications.

I apologise, but, in my opinion, you are not right. I suggest it to discuss. Write to me in PM.
Bravo, what necessary phrase..., a magnificent idea
Now all is clear, I thank for the information.