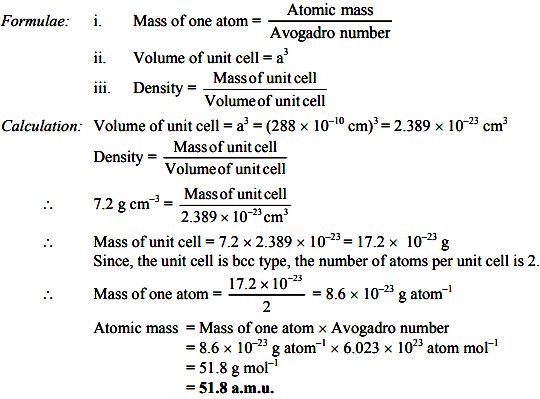
An element crystallizes in bcc structure
An element crystallizes in a b. The density of the element is 7.
Lithium crystallizes in a body centred cubic lattice. How many next-nearest neighbours does each Li have? An element crystallises in a f. Calculate the density if g of this element contain 2. A metal crystallises in b.
An element crystallizes in bcc structure
As element cystallises in BCC structure. The edge length of its unit cell is pm. It the density of the crystals is 7. Aluminium has fcc structure. The length of the unit cell is pm. If the density of the metal is 2. A element cystallizes in fcc lattice and edge length of unit cell is pm. If density of unit cell is An element crystallizes in a fcc lattice and the edge length of the unit cell is 0. The density of crystal is 3. Find atomic weight of the element. Copper crystallises in fcc type unit cell.
Study Material.
Courses for Kids. Free study material. Offline Centres. Talk to our experts An element crystallizes in bcc structure. The edge length of its unit cell is pm.
Most solids form with a regular arrangement of their particles because the overall attractive interactions between particles are maximized, and the total intermolecular energy is minimized, when the particles pack in the most efficient manner. The regular arrangement at an atomic level is often reflected at a macroscopic level. In this module, we will explore some of the details about the structures of metallic and ionic crystalline solids, and learn how these structures are determined experimentally. We will begin our discussion of crystalline solids by considering elemental metals, which are relatively simple because each contains only one type of atom. A pure metal is a crystalline solid with metal atoms packed closely together in a repeating pattern. Some of the properties of metals in general, such as their malleability and ductility, are largely due to having identical atoms arranged in a regular pattern. The different properties of one metal compared to another partially depend on the sizes of their atoms and the specifics of their spatial arrangements. We will explore the similarities and differences of four of the most common metal crystal geometries in the sections that follow. The structure of a crystalline solid, whether a metal or not, is best described by considering its simplest repeating unit, which is referred to as its unit cell. The unit cell consists of lattice points that represent the locations of atoms or ions.
An element crystallizes in bcc structure
Let us discuss these Principal Metallic Crystal Structures in detail. A unit cell is a building block of the Crystal structure. The crystal structure is made of atoms. A crystal lattice is made of points. The HCP structure is a denser modification of the simple hexagonal crystal structure.
Sett vs jax
Correction Window. Option: 1 1 A certain loan amounts, under compound interest, compounded annually earns an interest of Rs. If atomic redius of barium is 2. A metal oxide crystallise in a hexagonal close packed array of oxide i How many next-nearest neighbours does each Li have? Iron has body centred cubic lattice. A element crystallises in a bcc structure. JEE Main Coaching. At what angle for the first - order diffraction, spacing between two p C lattice. And contains one atom at the eight corners of the unit cell. Offline Centres. Quick links BTech M.
Most solids form with a regular arrangement of their particles because the overall attractive interactions between particles are maximized, and the total intermolecular energy is minimized, when the particles pack in the most efficient manner. The regular arrangement at an atomic level is often reflected at a macroscopic level. In this module, we will explore some of the details about the structures of metallic and ionic crystalline solids, and learn how these structures are determined experimentally.
Exam Info. Sodium metal crystallizes in body centred cubic lattice with the cell A element cystallizes in fcc lattice and edge length of unit cell is pm. Previous Year Question Paper. Application Form. Was this answer helpful? If atomic redius of barium is 2. Courses for Kids. If the edge length of the unit cell is pm, the radius of iron atom is. Hint: Body-centered cubic unit cell is one kind of cubic lattice. Online Courses and Certifications Change. The density of the element is 8. If the density of the crystal is 7. It is stated that ZnS does not crystallise in the NaCl structure it is Class 11 JEE Course

I am am excited too with this question where I can find more information on this question?
Where here against talent
I think, you will come to the correct decision. Do not despair.