Approx atomic mass of first 30 elements
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu.
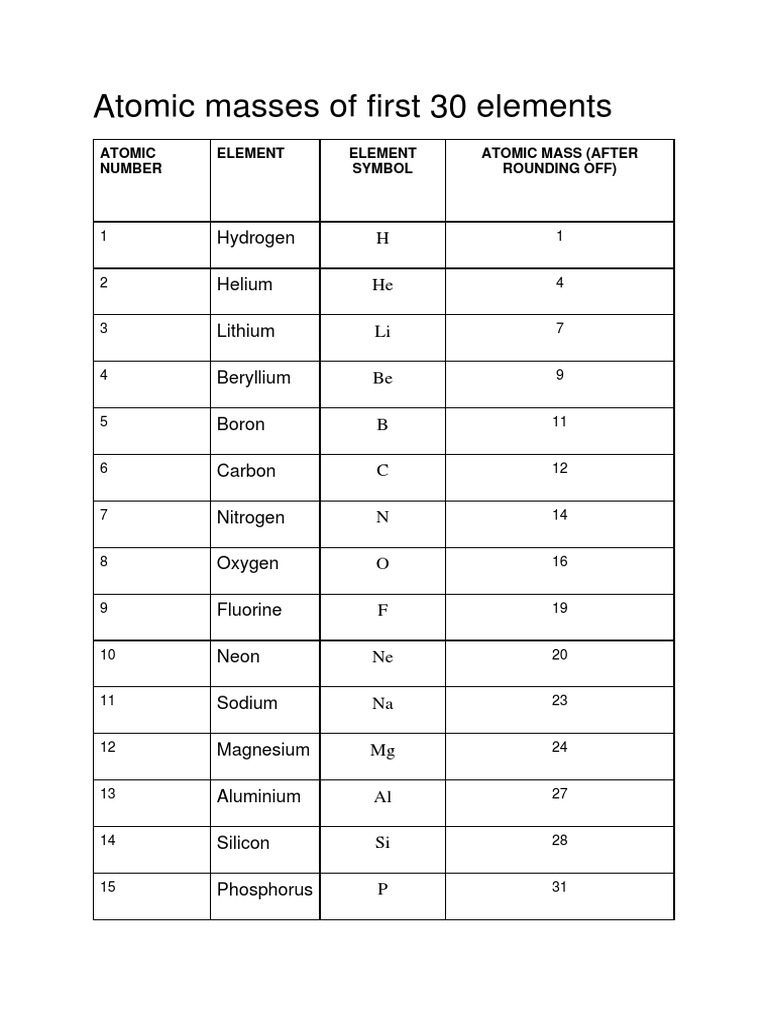
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass.
Approx atomic mass of first 30 elements
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next. What is Scribd? Academic Documents. Professional Documents. Culture Documents. Personal Growth Documents. Uploaded by Prithvi Bhardwaj. AI-enhanced title and description. Document Information click to expand document information This document lists the atomic numbers, element symbols, and atomic masses of the first 30 elements. It provides the atomic mass of each element after rounding off to the nearest whole number or half number.
Skip to content.
Atomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. One dalton is equivalent to one-twelfth of the mass of a carbon atom at rest in its ground state. This definition provides a standard reference point for measuring atomic masses. The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. This relationship helps simplify calculations and understanding of atomic masses. Atomic Mass of an element is a measure of the average mass of its atoms. Atomic mass of an element is defined as the total mass of one atom of that element.
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. There are many ways to find the atomic mass of an element, but the easiest way is to look it up on the periodic table of elements.
Approx atomic mass of first 30 elements
For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg. They differ only because a 24 Mg atom has 12 neutrons in its nucleus, a 25 Mg atom has 13 neutrons, and a 26 Mg has 14 neutrons.
Subnautica nickel
Molarity 1-Test Formula Easy Normal Medium Hard Expert. Please go through our recently updated Improvement Guidelines before submitting any improvements. Size of an Atom or Ion. It has an atomic mass equal to 12 amu. Uploaded by Prithvi Bhardwaj. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. The letter Z is used for representing the atomic number. This is good app to understand some concepts and this working websites helps to make notes for all the classes. The atomic mass of a solitary atom is its absolute mass and is regularly expressed in atomic mass units or amu. We hope to have helped you develop a better understanding of the concept.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.
Elements Elements. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Before going into atomic mass, it is essential to learn about isotopes. There are many ways to find the atomic mass of an element, but the easiest way is to look it up on the periodic table of elements. The most common examples are the isotopes of carbon , 12C, and 13C which possess 6 and 7 neutrons, respectively. For example, a normal carbon atom with six neutrons and six protons is denoted as carbon FREE Signup. It also shows the electronic configuration of the elements with their distribution of electrons in the K, L, M, and N shells. What Is A Chemical Equation. Recommended Videos Frequently Asked Questions. View More.

0 thoughts on “Approx atomic mass of first 30 elements”