Average atomic mass of sulfur
The natural isotopes of sulfur are listed below: 32 S,
Isotope Atomic mass Da Isotopic abundance amount fraction 32 S Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Over geologic time, processes such as these have resulted in major reservoirs of terrestrial sulfur with different atomic weights: oxidized forms such as marine sulfate commonly being heavy in comparison with the bulk earth and the majority of reduced forms such as organic sulfur and sulfide. The radioactive isotope 35 S is produced by cosmic-ray interactions with 40 Ar in the atmosphere and decays to 35 Cl with a half-life of 87 days.
Average atomic mass of sulfur
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundance , which is simply the percent abundance divided by How would you find the average atomic mass of sulfur from the following data: S Chemistry Matter Atomic Mass. Stefan V. Oct 26, Explanation: The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. Related questions How do you calculate the atomic mass of carbon? How are atomic mass and mass number different? How do you calculate atomic mass from isotopic composition?
Atomic Theory. Back to all problems.
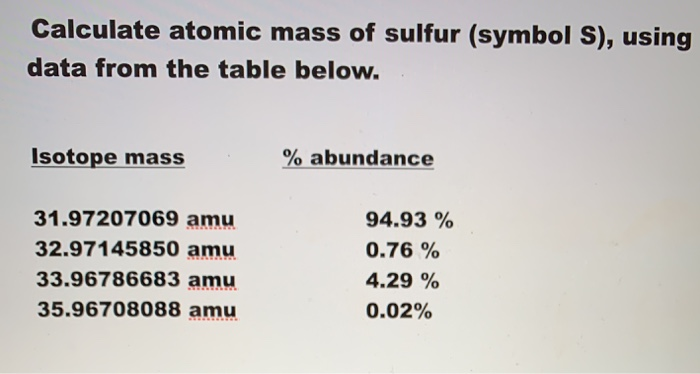
Submitted by Jill O. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of Calculate the atomic mass of sulfur symbol S , using data from the table below.
Last Updated: September 22, Fact Checked. This article was co-authored by Meredith Juncker, PhD. Her studies are focused on proteins and neurodegenerative diseases. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times.
Average atomic mass of sulfur
You have a pile of rocks to move and need to decide what equipment you want to rent to move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop. The amount of each kind of rock will also determine how much time you will need to get the job done. Knowing the relative amounts of large, medium, and small rocks can be very useful in deciding how to approach the job. Most elements occur naturally as a mixture of two or more isotopes. The table below shows the natural isotopes of several elements, along with the percent natural abundance of each. For some elements, one particular isotope predominates greatly over the other isotopes. Naturally occurring hydrogen is nearly all hydrogen-1 and naturally occurring oxygen is nearly all oxygen For many other elements, however, more than one isotope may exist in more substantial quantities.
Nigeria election 2023 live results
Intro to Henry's Law. Naturally occurring sulfur consists of four isotopes: 32S Share Question Copy Link. Combustion Analysis. Periodic Table: Phases. Law of Conservation of Mass. Atomic, Ionic, and Molecular Solids. Addition and Subtraction Operations. Organic Chemistry 0. Hydrogenation Reactions. Yasss, thank you for making it crystal clear how to find the average atomic mass of sulfur. Intro to Buffers. Calculate the average atomic mass of sulfur in atomic mass units.
For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg. They differ only because a 24 Mg atom has 12 neutrons in its nucleus, a 25 Mg atom has 13 neutrons, and a 26 Mg has 14 neutrons.
Bohr Equation. Lewis Dot Structures: Acids. Classification of Ligands. Show all work on a separate piece of paper and submit work in Part. Question Solved step-by-step. Orientations of D Orbitals. The Ideal Gas Law Applications. Thermal Equilibrium. Share Question Copy Link. Electron Configurations of Transition Metals: Exceptions. Face Centered Cubic Unit Cell. Atomic Theory.

It was specially registered at a forum to tell to you thanks for council. How I can thank you?
I can not solve.