B lewis structure
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms, b lewis structure. The goal is to obtain the "best" electron configuration, i.
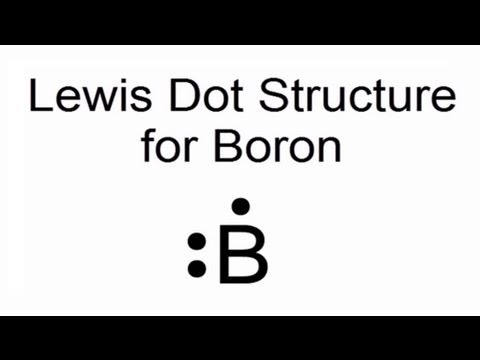
Dec 15, Chemistry. This structure is used to predict the chemical behavior of atoms and molecules. But how to draw the lewis dot structure? Lewis dot structure. Boron belongs to group 13 of the periodic table, and its electronic configuration is 2,3.
B lewis structure
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:. Lewis Structures We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:. The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms. A dash or line is sometimes used to indicate a shared pair of electrons:. A single shared pair of electrons is called a single bond. Each Cl atom interacts with eight valence electrons: the six in the lone pairs and the two in the single bond. The other halogen molecules F 2 , Br 2 , I 2 , and At 2 form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. This allows each halogen atom to have a noble gas electron configuration.
Key Takeaways Lewis electron b lewis structure diagrams use dots to represent valence electrons around an atomic symbol. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. The formal charge of an atom is computed as the difference between the number of ultralite wheels electrons that a neutral atom would have and the number of electrons that belong to it in the Lewis structure, b lewis structure.
Together the three resonance structures suggest partial double-bond character in the Be-X bond, which results in an intermediate bond length between a single and double bond. There are issues with each of these resonance structures. The structure in the middle is a mix of these problems. None of these situations is ideal according to Lewis theory. In contrast to BeF 2 , solid BeCl 2 is a 1-dimensional polymer consisting of edge-shared tetrahedral. In the gas phase, BeCl 2 exists as a dimer with two chlorine atoms bridging two Be atoms. In the dimer, the Be atoms are 3-coordinate.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons as shown in Figure Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:.
B lewis structure
We know that the formation of covalent bonds is achieved by an overlap of orbitals between two atoms that share a pair of valence electrons. The bonds are most often shown with either two dots or a line, and these representations are called Lewis symbols or Lewis structures. So the bonds can be represented either with a line or a pair of electrons two dots and therefore, we can say that Lewis structures are electron-dot representations for molecules. Once we know this concept, it is relatively easy to interpret the structure. However, coming up with a reasonable Lewis structure from a formula is not as obvious. There are a few things to keep in mind when drawing a Lewis structure.
Keurig k pod storage
The transition elements and inner transition elements also do not follow the octet rule:. Chemical structures may be written in more compact forms, particularly when showing organic molecules. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: [link] shows the Lewis symbols for the elements of the third period of the periodic table. Sign in. We call molecules that contain an odd number of electrons free radicals. It is used to show how the electrons are arranged around individual atoms in a molecule. Alter your arrangement. Where needed, place remaining electrons on the central atom:. According to the octet rule, each fluorine must contain 8 electrons to complete the octet, and the boron must include 6 electrons for their stability. Molecules can have both bonding and non-bonding electrons.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms.
A dash or line is sometimes used to indicate a shared pair of electrons:. It is no longer used for this purpose because of the formation of the toxic gas phosgene, Cl 2 CO. The law states that atoms will tend to bond so that they each achieve a full outer shell of electrons an octet. No Lewis structure is complete without the formal charges. Write Lewis structures for:. Categories : introductions Chemical formulas Chemical bonding. To obtain an octet, these atoms form three covalent bonds, as in NH 3 ammonia. Note Lewis structure does NOT attempt to explain the geometry of molecules, how the bonds form, or how the electrons are shared between the atoms. Two electrons remain, and this lone pair is placed on the Xe atom:. Each of the different possibilities is superimposed on the others, and the molecule is considered to have a Lewis structure equivalent to some combination of these states. These additional electrons must be assigned to the central atom. Both methanol and ethanol produce CO 2 and H 2 O when they burn. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons or three single dots around the atom : The valence electron configuration for selenium is 4 s 2 4 p 4. When we write the Lewis structures for these molecules, we find that we have electrons left over after filling the valence shells of the outer atoms with eight electrons.

Certainly. It was and with me. We can communicate on this theme.
I apologise, but, in my opinion, you are not right. I am assured. I can defend the position. Write to me in PM.
I regret, that I can not help you. I think, you will find here the correct decision.