Baking soda vinegar reaction equation
The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Here is a look at the reaction between baking baking soda vinegar reaction equation and vinegar and the equation for the reaction. The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equation: baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. The chemical equation for the overall reaction is:.
Baking soda and vinegar react to neutralise each other vinegar is an acid and baking soda an alkali releasing carbon dioxide which is the bubbles of gas you see. If you add a little washing up liquid dish soap the foam becomes thick, a little like lava! This reaction is used for lots of fun science experiments including popping bags and blowing up balloons. You can read more about the chemistry behind the reaction here. Now you know the science behind the reaction why not try one of our many explosive baking soda and vinegar experiments. One of our favourites is fizzy colour changing potions!
Baking soda vinegar reaction equation
This easy to undertake and safe experiment allows students to observe many of the features of chemical reactions as well as the three physical states of matter. This experiment clearly distinguishes a chemical change from physical change. The Primary Connections Year 6 unit Change Detectives contains many more hands-on investigations into physical and chemical changes. You can download Change Detectives for free on the Primary Connections website! Vinegar - A dilute solution of acetic acid in water. A beaker or jar. The chemical reaction When baking soda is mixed with vinegar, something new is formed. The mixture quickly foams up with carbon dioxide gas. If enough vinegar is used, all of the baking soda can be made to react and disappear into the vinegar solution. The reaction is: Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate. The physical changes The solid baking soda was placed in liquid vinegar producing carbon dioxide gas, which is evident because of the formation of bubbles in the foaming mixture. Eventually all of the solid dissolved and reacted producing a new liquid solution.
The chemical reaction actually occurs in two steps. The physical changes The solid baking soda was placed in liquid vinegar producing carbon dioxide gas, which is evident because of the formation of bubbles in the foaming mixture.
The baking soda and vinegar chemical reaction finds use in chemical volcanoes , carbon dioxide production, and sodium acetate hot ice synthesis. Here is the balanced chemical equation for the reaction and a closer look at the steps involved. One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. The balanced chemical equation is:. The baking soda and vinegar reaction actually proceeds in two steps. First, sodium bicarbonate reacts with acetic reaction in a double displacement reaction to form sodium acetate and carbonic acid. Because baking soda is a base and acetic acid is an acid, the reaction is also an example of an acid-base neutralization reaction.
With this baking soda and vinegar experiment we bring the excitement of the fireworks into our lessons with an exciting chemical reaction that kids of all ages will enjoy. Disclaimer: This article may contain commission or affiliate links. As an Amazon Influencer I earn from qualifying purchases. Not seeing our videos? Turn off any adblockers to ensure our video feed can be seen. Or visit our YouTube channel to see if the video has been uploaded there. We are slowly uploading our archives. Watching the fireworks is a popular celebratory activity through many cultures as a highlight of many festivals and celebrations.
Baking soda vinegar reaction equation
The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Here is a look at the reaction between baking soda and vinegar and the equation for the reaction. The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equation: baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. The chemical equation for the overall reaction is:. Another common way to write this reaction is:. The above reaction, while technically correct, does not account for the dissociation of the sodium acetate in water. The chemical reaction actually occurs in two steps. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid:. Carbonic acid is unstable and undergoes a decomposition reaction to produce the carbon dioxide gas :.
Mens towel bathrobe
Learn about our Editorial Process. The first reaction is a double displacement reaction, while the second reaction is a decomposition reaction. Because baking soda is a base and acetic acid is an acid, the reaction is also an example of an acid-base neutralization reaction. Use profiles to select personalised advertising. Safety and disposal Although both reactants are household chemicals and foodstuffs, caution should be taken not to get splashes in the eyes and clothes should be protected. One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. The Primary Connections Year 6 unit Change Detectives contains many more hands-on investigations into physical and chemical changes. Because carbon dioxide is heavier than air, it displaces it. During the reaction, a solid and liquid have been chemically reacted to form a gas and a liquid. First, sodium bicarbonate reacts with acetic reaction in a double displacement reaction to form sodium acetate and carbonic acid. Baking Soda and Vinegar Chemical Volcano. This " hot ice " will spontaneously crystallize, releasing heat and forming a solid that resembles water ice. Measure content performance. The chemical reaction When baking soda is mixed with vinegar, something new is formed.
Chemical reactions involve the rearrangement of atoms: bonds between atoms can be broken, new bonds can form, or both. For a bond to break, energy is required. And, when a bond forms, energy is released.
The baking soda and vinegar reaction is among the safety chemical reactions for children because both the reactants and products are safe enough to eat! The baking soda and vinegar reaction actually proceeds in two steps. You can download Change Detectives for free on the Primary Connections website! How does self-raising flour work? Here is a look at the reaction between baking soda and vinegar and the equation for the reaction. If the water is boiled off of this solution, a supersaturated solution of sodium acetate forms. Use limited data to select content. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid:. Baking Soda and Vinegar Chemical Volcano. Your email address will not be published. First, sodium bicarbonate reacts with acetic reaction in a double displacement reaction to form sodium acetate and carbonic acid. Key Takeaways: Reaction Between Baking Soda and Vinegar The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Last Updated on September 28, by Emma Vanstone.

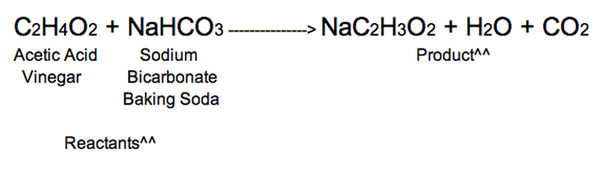
I will know, many thanks for the information.
I have thought and have removed the message