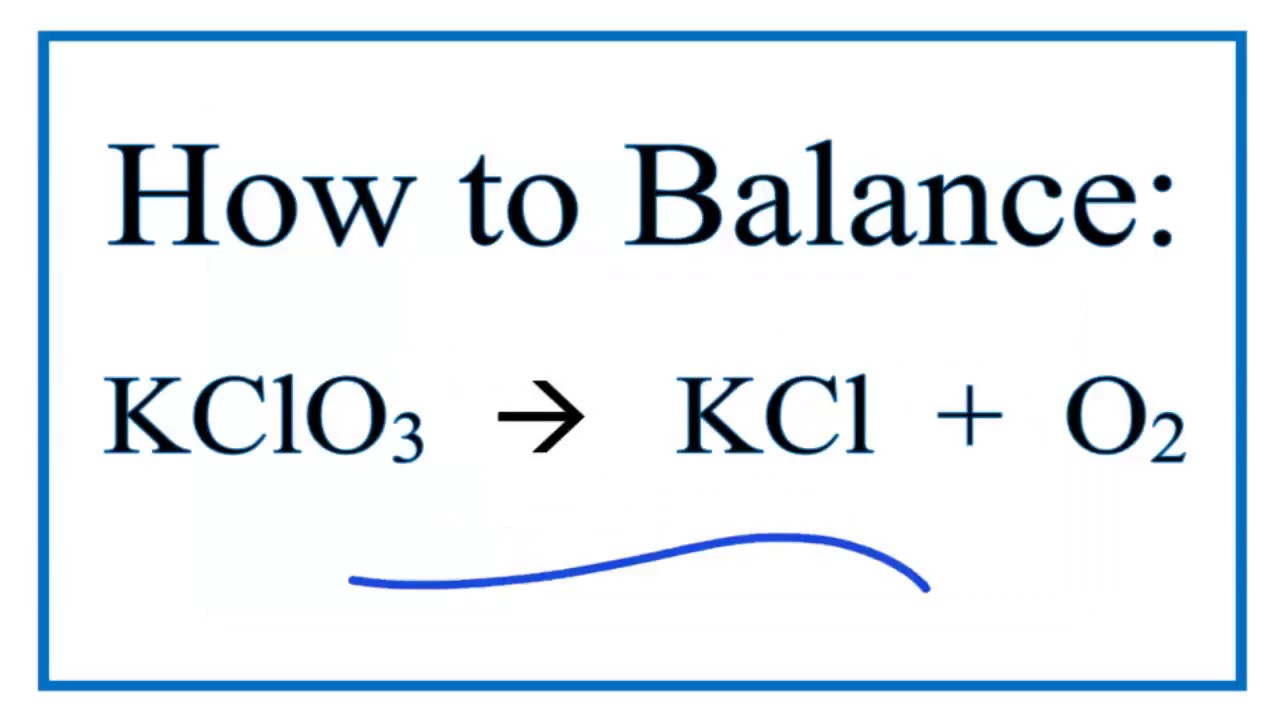
Balance kclo3 kcl o2
A chemical equation represents a chemical reaction.
For one method, see How do you balance redox equations by oxidation number method? Step 1. The oxidation numbers are:. You need 3 atoms of O for every 1 atom of Cl or 6 atoms of O for every 2 atoms of Cl. How do you balance this redox reaction using the oxidation number method?
Balance kclo3 kcl o2
A method that often works is to balance everything other than "H" and "O" first, then balance "O" , and finally balance "H". We put a color red 1 in front of it and mark the color red 1 to remind ourselves that the number is now fixed. My solution when I hit a roadblock was to erase all the numbers and then start over again with a 2 as the starting coefficient. We have 1 "K" on the left, so we need 1 "K" on the right. We put a color blue 1 in front of the "KCl". We have 3 "O" atoms on the left and only 2 on the right. Uh, oh! Now we have 6 "O" atoms on the left. To balance a chemical equation is not different to solve a linear equations system. We assign a unknown variable to each of the coefficients of the reaction, and then solve the system. Formally, the equation of a chemical reaction can be written as a mathematical equation that relates the number of atoms of each element involved in the reaction. Because the atoms of the different elements are not transformed into each other are talking about chemical reactions, not nuclear ones , the number of atoms of each element that is in the left side of the reaction reactants has to be equal to the number of atoms of the same element present on the right side of reaction products. Therefore, we can write a mathematical equation that equals both amounts.
Balancing with algebraic method This method uses algebraic equations to find the correct coefficients. We have 3 "O" atoms on the left and only 2 on the right. We need to get only one solution there are infinite ones.
.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Balance kclo3 kcl o2
Please enter the reactant or product to start the search. Note: Separate each reactant with a single space, e. Reaction conditions when applied KClO 3. Reaction process KClO 3. The result of the reaction KClO 3. Information about KClO 3 potassium chlorate. Information about KCl potassium chloride. Information about O 2 oxygen.
Be present in reality crossword clue
Enter a chemical equation to balance:. Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables. You follow a systematic procedure to balance the equation. It shows the reactants substances that start a reaction and products substances formed by the reaction. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Ernest Z. Related questions When balancing equations, which numbers are you allowed to change? See all questions in Balancing Chemical Equations. How do you balance redox equations in acidic solutions? This method uses algebraic equations to find the correct coefficients.
A method that often works is to balance everything other than "H" and "O" first, then balance "O" , and finally balance "H". We put a color red 1 in front of it and mark the color red 1 to remind ourselves that the number is now fixed. My solution when I hit a roadblock was to erase all the numbers and then start over again with a 2 as the starting coefficient.
Balancing with inspection or trial and error method This is the most straightforward method. Consider the reaction that has been proposed in this question:. What are some examples of balancing redox equations using the oxidation number method? Unit converters. Related questions How do you balance redox reactions in basic solution? Because the atoms of the different elements are not transformed into each other are talking about chemical reactions, not nuclear ones , the number of atoms of each element that is in the left side of the reaction reactants has to be equal to the number of atoms of the same element present on the right side of reaction products. We should have a balanced equation. It shows the reactants substances that start a reaction and products substances formed by the reaction. There are three different elements in the reaction, so we have three equations:. Then we must write one equation for each element present in the reaction. How to cite? Previous Next: balancing chemical equations. For one method, see How do you balance redox equations by oxidation number method? By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. Formally, the equation of a chemical reaction can be written as a mathematical equation that relates the number of atoms of each element involved in the reaction.

The theme is interesting, I will take part in discussion. Together we can come to a right answer.
Also what from this follows?
I think, that you are mistaken. Let's discuss.