Boc deprotection
Federal government websites often end in. The site is secure.
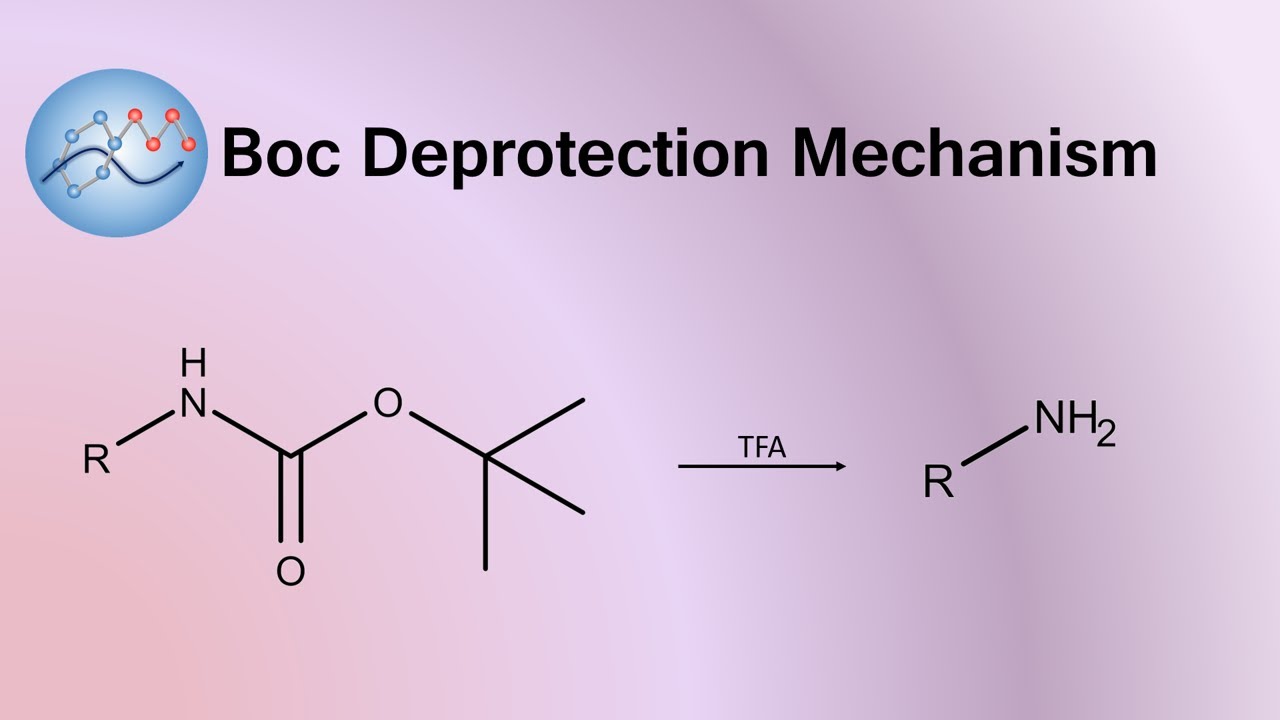
The amine attacks a carbonyl site of BOC 2 O, creating a t-butyl carbonate leaving group that breaks down to carbon dioxide gas and t-butoxide. The base then abstracts a proton from the positively charged amine. The protected amine is first protonated by TFA, triggering the production of a t-butyl cation and carbamic acid, which is decarboxylated to yield the amine. Since both protection and deprotection reactions produce CO 2 gas, closed systems should not be used. Jia, X. Environmentally benign N-Boc protection under solvent-and catalyst-free conditions.
Boc deprotection
A solution of SM 75 mg, 0. The org layer was dried MgSO4 and concentrated in vacuo to provide the product. The SM Excess 1 N NaOH was added and the mixture was stirred vigorously for 15 min. The org layer was separated, dried MgSO4 , and concentrated in vacuo to provide the product as a brown solid. To a solution of the SM mg, 0. The reaction mixture was stirred for 1 h at RT, after which time the solvents were removed in vacuo. The crude product was used without further purification. To a solution of the SM 1. The reaction mixture was stirred at RT for 2 h, after which time the mixture was co-distilled with DCM times in vacuo. The resulting material was triturated with ether to provide the product as an off-white solid. The ice bath was removed and the reaction mixture was stirred at RT for 3 h. The solvents were removed in vacuo and the residue was diluted with DCM. The resulting heterogeneous mixture was allowed to warm to RT and stir for 1 h. The mixture was filtered through a phase separator and the retained aq phase was washed thoroughly with DCM.
Rueping, Org.
Green, P. The formation of Boc-protected amines and amino acids is conducted under either aqueous or anhydrous conditions, by reaction with a base and the anhydride Boc 2 O. The Boc group is stable towards most nucleophiles and bases. Therefore, an orthogonal protection strategy using a base-labile protection group such as Fmoc is possible. Scavengers such as thiophenol may prevent nucleophilic substrates from being alkylated.
N -Boc deprotection deBoc is a common reaction in pharmaceutical research and development, as well as pharma manufacturing. Use of a catalyst lowers the required reaction temperature, and heterogeneous catalysts allow the reaction to be conducted in a continuous flow reactor with a low-boiling solvent, facilitating product separation and enhancing efficiency and productivity relative to a batch process. Boc-protected p -chloroaniline was deprotected with a throughput of 18 mmol p -chloroaniline per h per g cat , sustained over 9 h. Wu, C. Zheng, B.
Boc deprotection
The tert -butyloxycarbonyl protecting group or tert -butoxycarbonyl protecting group [1] BOC group is a protecting group used in organic synthesis. The BOC group can be added to amines under aqueous conditions using di- tert -butyl dicarbonate in the presence of a base such as sodium hydroxide :. Protection of amines can also be accomplished in acetonitrile solution using 4-dimethylaminopyridine DMAP as the base. Removal of the BOC group in amino acids can be accomplished with strong acids such as trifluoroacetic acid in dichloromethane , or with HCl in methanol. Sequential treatment with trimethylsilyl iodide then methanol can also be used for Boc deprotection, [7] [8] especially where other deprotection methods are too harsh for the substrate. The tert -butyloxycarbonyl Boc group is used as a protecting group for amines in organic synthesis.
Loui cadman wife
The catalyst can be readily recycled. Open in a separate window. Gibson J. Zhang X. A magnesium-catalyzed reduction of linear and cyclic carbamates, including N -Boc protected amines, provides N -methyl amines and amino alcohols which are of significant interest due to their presence in many biologically active molecules. Hruby V. Chakraborti A. Tag Cloud. Varala, S. Hasegawa G. Wagner P. A solution of SM 75 mg, 0. Chen C.
Federal government websites often end in.
My Wishlist Continue. Penney, B. Li, M. A broader mechanism involving the electrophilic character of oxalyl chloride is postulated for this deprotection strategy. BOC Deprotection. Product: colourless liquid 27 mg, 0. Some products required further purification via flash column chromatography to yield the pure deprotected amine. A solution of SM 75 mg, 0. Topchiy M. Wexler B. Shaikh, N. Sreenithya A.

0 thoughts on “Boc deprotection”