Br valence electrons
Wiki User. The element bromine has 35 protons in its nucleus, and therefore in its electrically neutral state it also has 35 electrons. Two electrons fit in the innermost shell, eight fit in the next shell, eighteen fit in the next shell, which takes the total up br valence electrons If we subtract 28 from 35 we get seven, voila.
The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a 1- bromide ion, it would have to gain an additional electron. Below is the Lewis dot structure for a neutral bromine atom, which has seven valence electrons. The extra valence electron gives it a negative charge. The diagram below shows how a bromine atom gains an electron from the element lithium in order to form the ionic compound LiBr. Chemistry Electron Configuration Electron Configuration.
Br valence electrons
An element has the electronic configuration 1 s 2 , 2 s 2 2 p 6 , 3 s 2 3 p 2. Use app Login. In the electron configuration of Br , where are the valence electrons? Are they in the 3d? Open in App. Verified by Toppr. Explanation : When the five 3d orbitals have 10 electrons this subshell is filled. Being filled the d subshell becomes stable and falls into the lower third electron shell. The 4s and 3d are similar in the kinetic energy required to occupy these orbitals. The 3d is closer to the nucleus but the 3d orbitals are more complicated with up to four lobes. The 4s is farther from the nucleus but is a simple spherical orbital.
In order for a bromine atom to become a 1- bromide ion, it would have to gain an additional electron. Molecular Formula. Emission Spectrum.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the Visual Elements image see the Uses and properties section below. Group A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. Block Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp s , principal p , diffuse d , and fundamental f. Atomic number The number of protons in an atom.
Br valence electrons
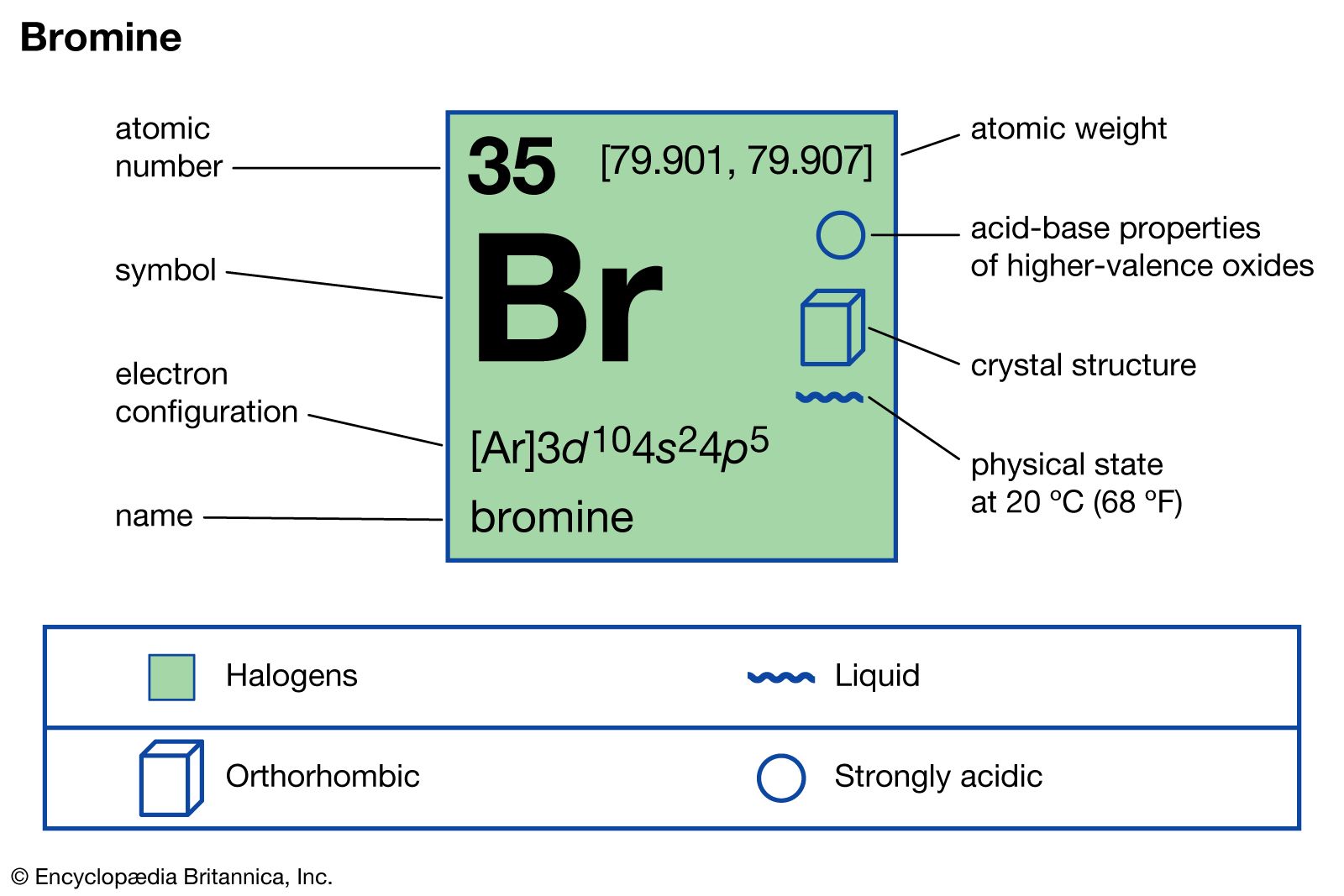
Get a thorough understanding of Bromine valence electrons here in the article. You will further explore the elements with its others significant properties. In chemistry, Bromine is a chemical element with its atomic number It has the writing symbol as Br in the periodic table. Bromine belongs to the group of halogen and hence has similar properties. Being the halogen element Bromine can easily evaporate at a given room temperature.
Rhea ripley
What is the valency of bromine What is the valency of bromine? Addition and Subtraction Operations. Being filled the d subshell becomes stable and falls into the lower third electron shell. Acids Introduction. Test for Ions and Gases. If we subtract 28 from 35 we get seven, voila. Equilibrium Constant K. Band of Stability: Beta Decay. Intro to Henry's Law. De Broglie Wavelength. Intro to Addition Reactions. The valency for bromine is
Bromine Electron Configuration : Bromine Br is a chemical element. The atomic number of bromine is It is the fuming red-brown liquid at room temperature and the third-lightest halogen.
Explanation: The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Amphoteric Species. Solubility Rules. Molecular Orbital Theory. Heating and Cooling Curves. Related questions How do electron configurations in the same group compare? Naming Other Substituents. Solutions: Mass Percent. Chemical Bonds. Instantaneous Rate. Paramagnetism and Diamagnetism. How many electrons on their outer shell does bromine have?

It agree, this rather good idea is necessary just by the way
It is very a pity to me, that I can help nothing to you. But it is assured, that you will find the correct decision.