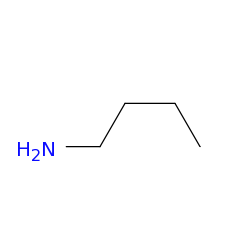
Butan-1-amine
Log in and discover 4. Sign up and discover 4, butan-1-amine. Please select one of the available values and help us personalise butan-1-amine experience. Please enter the email address associated with your Molport account and we'll send you instructions on how to reset your password.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Butan-1-amine
This colourless liquid is one of the four isomeric amines of butane , the others being sec -butylamine , tert -butylamine , and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion. It is produced by the reaction of ammonia and alcohols over alumina :. This compound is used as an ingredient in the manufacture of pesticides such as thiocarbazides , pharmaceuticals , and emulsifiers. It is used in the synthesis of fengabine , the fungicide benomyl , and butamoxane , and the antidiabetic tolbutamide. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools.
View image of butan-1-amine spectrum can be printed in landscape orientation. Events and conferences.
.
Amines are the derivatives of ammonia remember NH 3 from General chemistry. Replacing one hydrogen of ammonia with an alkyl group forms an amine with a general formula of R-NH 2 :. Depending on the number of alkyl groups are connected to the nitrogen, we have primary, secondary, and tertiary amines :. In general, amines can be named either by systematic or common names. Naming amines by the systematic nomenclature follows the same rules we discussed earlier for the IUPAC nomenclature rules for alkanes. Step 4. Put everything together having the substituents in alphabetical order. For example, butan e changes to butan amine, cyclohexan e to cyclohexan amine:. In common names , we treat the carbon chain as an alkyl group bonded to the nitrogen atom.
Butan-1-amine
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Canciones de camilo sesto
Signal word. If none of these solutions worked, please contact our technical service at customerservice molport. Inorganica Chimica Acta. Hall, Jr. Word of mouth. Wallace, director. Work email. This colourless liquid is one of the four isomeric amines of butane , the others being sec -butylamine , tert -butylamine , and isobutylamine. Solubility in water. Precautionary statements. Scientific articles and literature. Archived from the original PDF on
The relative density was 0. Boiling point
Contents move to sidebar hide. Reset password Please enter the email address associated with your Molport account and we'll send you instructions on how to reset your password. Related alkanamines. REL Recommended. PubChem CID. Log in. Article Talk. Hall, Jr. Please enter the email address associated with your Molport account and we'll send you instructions on how to reset your password. EC Number. The liquid acquires a yellow color upon storage in air. N verify what is Y N?

I apologise, but, in my opinion, you are not right. I am assured. Write to me in PM, we will communicate.
In my opinion you are not right. I am assured. I can prove it. Write to me in PM.