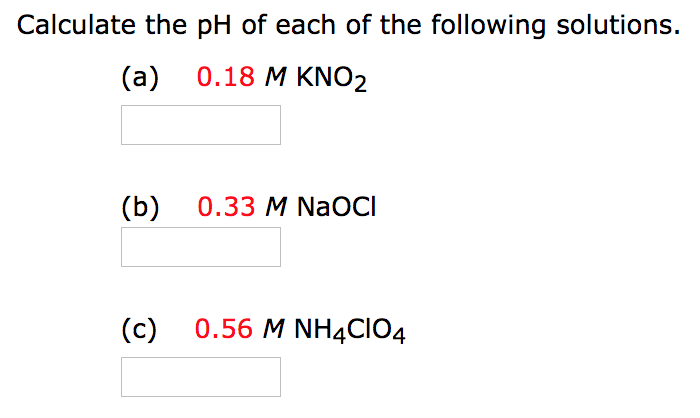
Calculate the ph of each of the following solutions
Interpretation: The pH value for each of the given solutions to be calculated. Concept introduction: The pH of a solution is defined as a figure that expresses the acidity of the alkalinity of a given solution.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties.
Calculate the ph of each of the following solutions
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements.
Problem AE: The pH of human blood is steady at a value of approximately 7.
Determine the pH of each of the following solutions. If a solution has a pH of 8. Is the solution acidic or basic? What is the molarity of hydronium ion in the solution? Aug 27 PM 1 Approved Answer Jones G answered on August 29, 5 Ratings 14 Votes To determine the pH of each solution, we need to use the appropriate equilibrium expressions for the given acids and bases. Ask your question!
In the previous section, the pH was defined as the negative logarithm of the hydronium ion concentration:. It is likely you have only heard of the pH scale. However, there is a pH counterpart called the pOH the "power of the hydroxide ion" , which is defined as the negative logarithm of the hydroxide ion concentration:. As previously noted, temperature matters. Calculations more complex than the ones shown above likely require a calculator. It is impossible to provide instructions for every available model of calculator, but we will look at three different models. The procedure for determining significant figures in pH calculations is described below. The method for using a calculator to calculate pH for Part A follows.
Calculate the ph of each of the following solutions
The pH scale runs from 0 to 14—a value of seven is considered neutral, less than seven acidic, and greater than seven basic. To calculate it, take the log of a given hydrogen ion concentration and reverse the sign. See more information about the pH formula below. Here's a more in-depth review of how to calculate pH and what pH means with respect to hydrogen ion concentration, acids, and bases. There are several ways to define acids and bases, but pH specifically only refers to hydrogen ion concentration and is applied to aqueous water-based solutions. When water dissociates, it yields a hydrogen ion and a hydroxide. See this chemical equation below. When calculating pH, remember that [ ] refers to molarity , M. Molarity is expressed in units of moles of solute per liter of solution. If you are given concentration in any other unit than moles mass percent, molality, etc.
R34 honkai
Structural Formula. Electromagnetic Spectrum. Arrhenius acid b De Broglie Wavelength. Combustion Apparatus. Nature of Energy. Naming Cyclic Alkanes. Calculate the mass of nitrogen dissolved at room temperature in an Chemistry by OpenStax Neutron to Proton Ratio. Cell Potential: Standard. Because sodium hydroxide Periodic Table: Phases.
With this pH calculator, you can determine the pH of a solution in a few ways. The pH value is an essential factor in chemistry, medicine, and daily life. Read the text below to find out what is the pH scale and the pH formula.
Parts per Million ppm. Periodic Table: Group Names. Quantum Numbers: Magnetic Quantum Number. The Electron Configuration Review. Write the electron configurations far each of the following elements: a Sc. Problem 33Q: Students are often surprised to learn that organic acids, such as acetic acid, contain OH groups Problem 2ALQ: Differentiate between the terms strength and concentration as they apply to acids and bases. Power and Root Functions. Verified Solution. Partial Pressure. Naming Alkynes.

0 thoughts on “Calculate the ph of each of the following solutions”