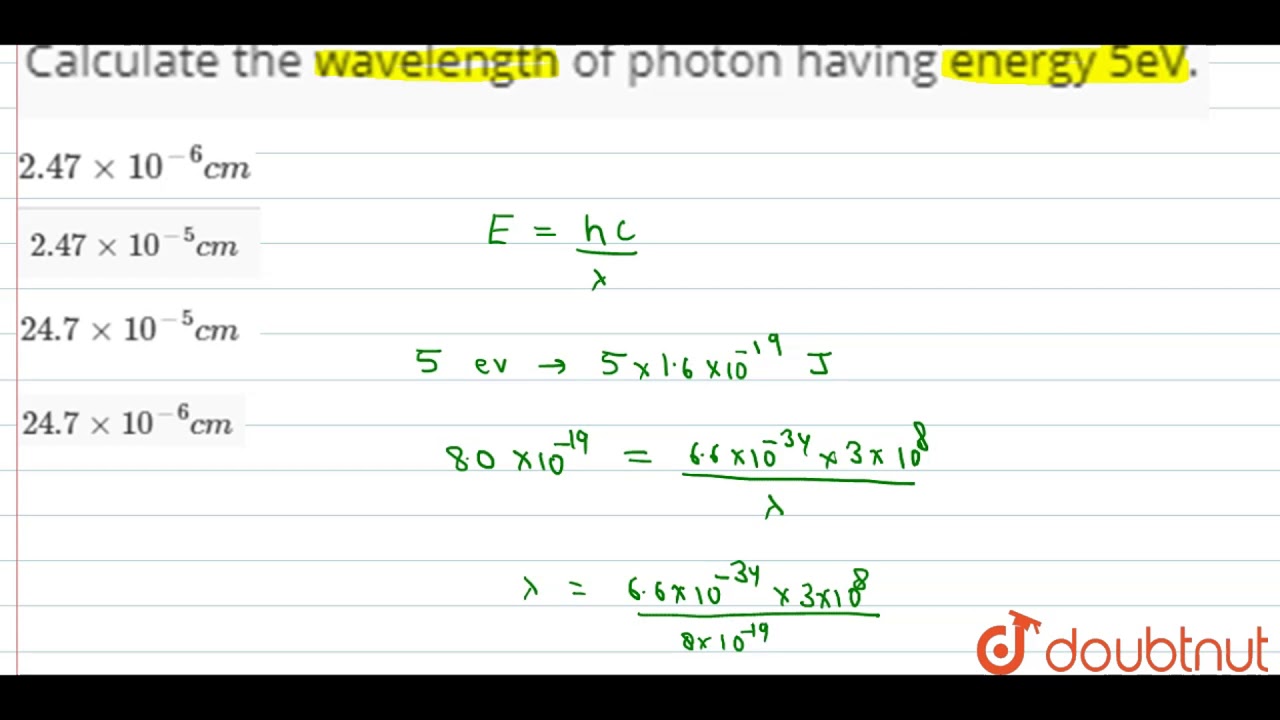
Calculate the wavelength of a photon
A ground state H atom absorbs a photon of energy 2. As the atom relaxes, it emits two photons: one of wavelength nm to reach an intermediate level, and a second to reach the ground state. What is the wavelength of the second photon emitted? The energy liberated by the electron falling back to the ground state must equal the energy absorbed, i.
Many, many problems in physics and chemistry require you to use an energy-to-wavelength calculator. Do you have an energy transition between two states and wonder what wavelength of light this corresponds to? Have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? If so, you've found the right place in Omni's energy-to-wavelength calculator, which will help you learn how to calculate wavelength from energy for a photon or wave! The energy to wavelength formula relies upon two other equations: the wave speed equation and the Planck—Einstein relation. The wave speed equation looks like this:. In most cases, we are looking at how a wave travels through a vacuum.
Calculate the wavelength of a photon
Last Updated: January 12, Fact Checked. This article was co-authored by Meredith Juncker, PhD. Her studies are focused on proteins and neurodegenerative diseases. There are 7 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 1,, times. Wavelength is the distance of 1 frequency wave peak to the other and is most commonly associated with the electromagnetic spectrum. If you know the speed and frequency of the wave, you can use the basic formula for wavelength. If you want to determine the wavelength of light given the specific energy of a photon, you would use the energy equation. Calculating wavelength is easy as long as you know the correct equation. This equation can also be used to determine the maximum wavelength of light necessary to ionize metals.
Do not change frequency when a wave enters a different medium. Have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength?
.
The wavelength of photons tells us about their energy. So in this article, we will look at what is the wavelength of photons and how to find it. Let us begin. Photons travel through electromagnetic waves. As the photon is ultimately a part of the electromagnetic wave, its wavelength will be the same as the electromagnetic wave. If the energy and frequency of a photon are known, then from that, one can easily find the wavelength of the photon. As the energy that is contained by photons is not divisible, they are often described as energy packets. Maxwell has described photons as electric fields which are traveling through space. Or, to put it another way, photon energy is stored in the form of an oscillating electric field that can oscillate at any frequency.
Calculate the wavelength of a photon
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. Introduction to electromagnetic waves. Properties of electromagnetic radiation and photons. Electromagnetic radiation is one of the many ways that energy travels through space. The heat from a burning fire, the light from the sun, the X-rays used by your doctor, as well as the energy used to cook food in a microwave are all forms of electromagnetic radiation. While these forms of energy might seem quite different from one another, they are related in that they all exhibit wavelike properties.
Hair rat
Create an account. Sample Wavelength Calculator. Nederlands: Golflengte berekenen. We then substitute this formula into the Planck-Einstein equation. How to. The speed of light never changes, so v will always equal 3. Download Article Explore this Article parts. Rearrange to solve for wavelength. Then rearrange the wave speed equation so that frequency is in terms of wavelength. Do not change frequency when a wave enters a different medium.
Last Updated: January 12, Fact Checked. This article was co-authored by Meredith Juncker, PhD.
The wavelength equals nm. Once you have rearranged the equation, you can solve for the wavelength by plugging in the variables for energy. Skip to Content. The difference between this and the absorbed energy will appear in the second emitted photon, which I found to be 1. Include your email address to get a message when this question is answered. Arts and Entertainment Artwork Books Movies. Ente Jan 2, Add comment. This can be seen if you examine the relationship between the two values - energy is inversely proportional to wavelength. By signing up you are agreeing to receive emails according to our privacy policy. Watch Articles. Updated: January 12, Check your work by calculating 4. Co-authored by:.

I consider, that you commit an error. I can prove it. Write to me in PM.
I think, that you are not right. I am assured. Let's discuss it. Write to me in PM.
It agree, the remarkable information