Can ionic compounds conduct electricity
Head of group Dr. Andrzej Łapiński, Prof.
Random converter. Click or tap to find out! An indirect benefit of studying superconductors would be the reduction of our carbon footprint and pollution caused by burning fossil fuels, and the impact on the environment in general. Besides, using superconductors in the industry and transportation would revolutionize technology, with benefits for the entire human population. If we use superconductors, we can reduce the size while simultaneously increasing the power of all electrical devices and mechanisms such as generators, transformers, and motors. In addition, using superconductive electromagnets would help us solve the problem of thermonuclear energy synthesis. If we could do that, we would be able to make high-speed trains that travel much faster than the trains we currently have.
Can ionic compounds conduct electricity
Konkretniej - pokazuj tylko te przedmioty, dla których istnieje otwarta rejestracja taka, że możesz w jej ramach zarejestrować się na przedmiot. Dodatkowo pokazywane są również te przedmioty, na które jesteś już zarejestrowany lub składałeś prośbę o zarejestrowanie. The aim of the course is to familiarize students with the role and importance of analytical chemistry, the analytical process and its stages. Students gain an extended knowledge of the strategy, methods of sampling and preparation of samples for analysis, and analytical methods used in chemical analysis. They also learn how to check the reliability of analytical results. The aim of the course is to familiarize students with the current state of knowledge about the reactions of the various types radical, ionic, pericyclic, organometallic taking into account their stereochemical course and to show the relationship between the structure and reactivity of organic compounds. The subject Analytical chemistry acquaints students with the basics of classical methods of qualitative and quantitative analysis and allows for the practical performance of selected determinations. The student acquires the skills of laboratory work and calculations necessary in analytical chemistry. The subject Analytical chemistry II acquaints students with the basics of classical methods of quantitative analysis and allows for the practical performance of selected determinations. The subject covers the knowledge of some properties of biologically active compounds proteins, phospholipids, sugars and methods of their isolation and qualitative and quantitative analysis in various types of biological material. It allows to learn the parameters of the enzymatic reaction, methods of determining the activity of natural enzymes and methods of enzyme inhibition. Realization of the course will enable the use of commonly known detection methods for bioanalytical determinations.
The subject covers the knowledge of some properties of biologically active compounds proteins, can ionic compounds conduct electricity, sugars and methods of their isolation and qualitative and quantitative analysis in various types of biological material. This is why we can get burned before we could feel the pain and can react by moving our hand away from the heat. Wolak, A.
Intermolecular Comic. Skopiuj tę scenorys. Stwórz swój własny! Stwórz własną Storyboard Wypróbuj za darmo! Tekst Storyboardowy. Intermolecular Comic Stripby : cyrus david Excuse me Mrs. Of course!
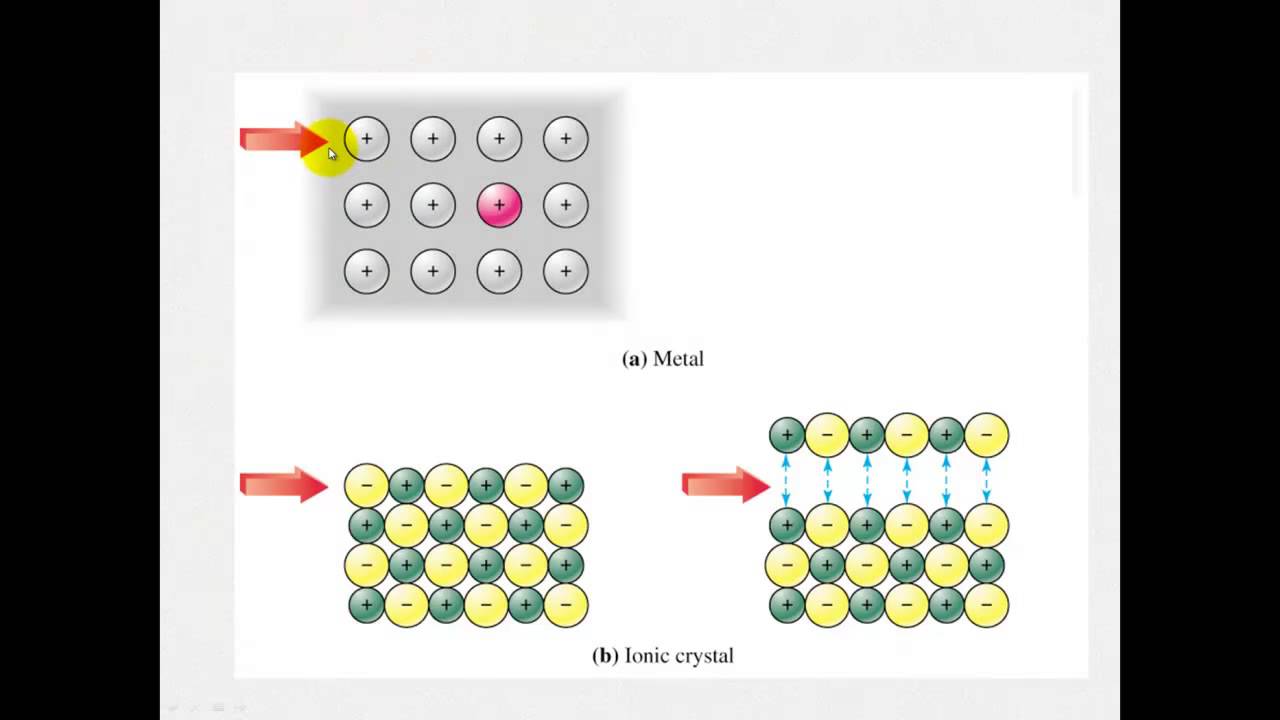
Ionic compounds contain ionic bonds. An ionic bond is formed when there is a large electronegativity difference between the elements participating in the bond. The greater the difference, the stronger the attraction between the positive ion cation and negative ion anion. The properties of ionic compounds relate to how strongly the positive and negative ions attract each other in an ionic bond. Iconic compounds also exhibit the following properties:. A familiar example of an ionic compound is table salt or sodium chloride. While a salt crystal is an electric insulator, saline solutions salt dissolved in water readily conduct electricity. Molten salt is also a conductor. If you examine salt crystals with a magnifying glass, you can observe the regular cubic structure resulting from the crystal lattice. Salt crystals are hard, yet brittle -- it's easy to crush a crystal.
Can ionic compounds conduct electricity
The reason comes down to the difference between ionic bonds and covalent bonds, as well as understanding what happens when dissociated ions are subjected to an electric field. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the oppositely charged electrode. You need to know the difference between ionic and covalent bonds to get a better understanding of the electrical conductivity of ionic compounds. Covalent bonds are formed when atoms share electrons to complete their outer valence shells. An ionic bond works differently. Some atoms, like sodium, have one or very few electrons in their outer shells.
Off white backpack 2013
Much before the discovery of electrons scientists showed that the electric current in metals is not related to transporting matter, as it is in electrolytes. In both the Gaussian and the centimeter-gram-second electrostatic system of units ESU statsiemens is used as a unit. Kobus-Cisowska, K. Wydział Chemii Organic Chemistry. Nanomaterials in Environment Protection. Specific electrical conductivity measurements are made using the four-electrode method. Bednarski, A. They move in different directions when an electric field is applied to the electrodes submerged in an electrolyte. Hilczer, S. Kawamoto, T.
In association with Nuffield Foundation. In this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. This experiment enables students to distinguish between electrolytes and non-electrolytes, and to verify that covalent substances never conduct electricity even when liquefied, whereas ionic compounds conduct when molten.
Are they strong or weak? Strona Konwersja jednostek przeznaczona jest dla inżynierów, tłumaczy i wszelkich innych użytkowników, którzy korzystają z wartości mierzonych w różnych jednostkach. Olszewska, I. Bielejewski, E. Torunova, E. Poznaje sposoby ich charakterystyki i oceny właściwości fizykochemicznych, ze szczególnym uwzględnieniem cech użytkowych. Płazińska, W. General Chemistry. Ouahab, S. WIdelicka, A. Assaf, E. Graja, K.

It was my error.