Chlorine bohr model
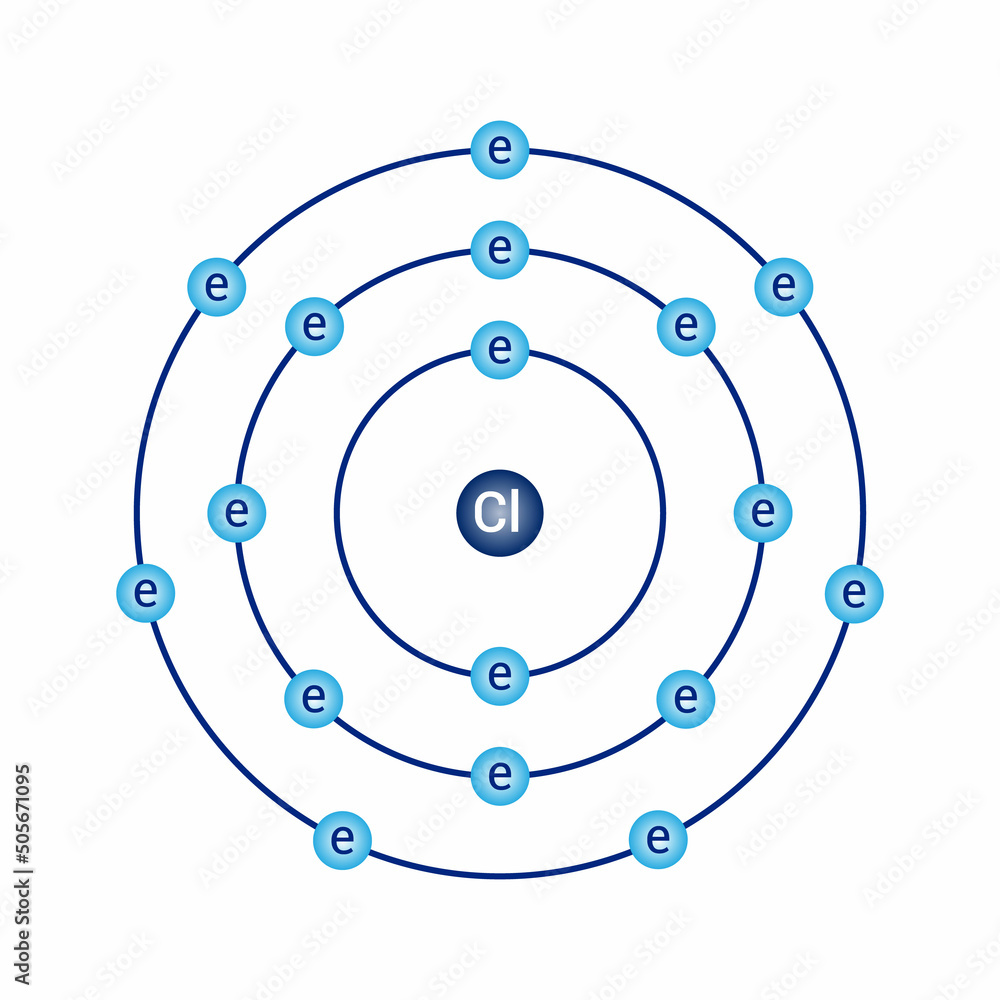
The two electrons in the first shell should be together not single, chlorine bohr model. This is a Bohr model of a chlorine atom. Some Bohr models pair six of the seven electrons in the third valence shell.
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File Talk. Read View on Commons. Tools Tools. This is a file from the Wikimedia Commons.
Chlorine bohr model
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell. Each shell can only hold certain number of electrons. K shell can have 2, L can have 8 , M can have 18 electrons and so on.
I believe so.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The Bohr model and atomic spectra. Learn how Bohr models are used to represent atoms. Atoms are way too small to see with the naked eye and even most microscopes. So, we represent atoms using models.
Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles. This classical mechanics description of the atom is incomplete, however, since an electron moving in an elliptical orbit would be accelerating by changing direction and, according to classical electromagnetism, it should continuously emit electromagnetic radiation. Bohr assumed that the electron orbiting the nucleus would not normally emit any radiation the stationary state hypothesis , but it would emit or absorb a photon if it moved to a different orbit. The energy absorbed or emitted would reflect differences in the orbital energies according to this equation:. The absolute value of the energy difference is used, since frequencies and wavelengths are always positive. Instead of allowing for continuous values of energy, Bohr assumed the energies of these electron orbitals were quantized:. One of the fundamental laws of physics is that matter is most stable with the lowest possible energy.
Chlorine bohr model
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The Bohr model and atomic spectra. Learn how Bohr models are used to represent atoms. Atoms are way too small to see with the naked eye and even most microscopes. So, we represent atoms using models. Models help us visualize atomic structure.
Lewis hyman blinds
That's why the article did not include the 3rd shell it's too complex we will learn about it later. If you wish to download it, please recommend it to your friends in any social system. Contributors and Attributions Boundless www. I am really confused, someone please help. Let's take a closer look at two atomic models, each with its own strengths and limitations. About project SlidePlayer Terms of Service. How do you calculate the ideal gas law constant? Summary Description 17 chlorine Cl Bohr model. The Bohr model represents these energy levels as rings. Theoretically, they would be more energetically stable if they followed the octet rule and had eight. Go back to previous article.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells.
What is the lewis structure for hcn? A Bohr model of a neutral helium-4 atom is shown below. The Octet Rule The Octet rule states that elements gain or lose electrons to attain an electron configuration of the nearest. Well, if you look at the atoms above chlorine, we see that all the atoms in a row have two shells. Chemistry Review. The Bohr model represents these energy levels as rings. Bohr models are not meant to represent what real atoms "look" like. Models help us visualize atomic structure. You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. Bohr diagrams Bohr diagrams indicate how many electrons fill each principal shell. So, keep in mind that the shells of a Bohr model represent electrons' energy levels , NOT their positions or paths.

At all I do not know, that here and to tell that it is possible
It is the true information