Cl2 lewis
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them, cl2 lewis. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence cl2 lewis in a Cl2 chlorine moleculefirst of all you should know the valence electrons present in a single chlorine atom.
Ready to learn how to draw the lewis structure of Cl2? Here, I have explained 6 simple steps to draw the lewis dot structure of Cl2 along with images. Lewis structure of Cl2 Chlorine contains one single bond between both the Chlorine Cl atoms. And both the Chlorine atoms have three lone pairs on it. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
Cl2 lewis
Chlorine gas, a member of the halogen group, exists in the form of a diatomic molecule with the chemical formula Cl 2. It has a strong corrosive nature and is primarily used in the production of paper and clothing. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell. By dividing the total valence electrons by two, we find the total electron pairs. For the Cl 2 molecule, there are seven electron pairs in their valence shells. To get the best Lewis structure, reduce charges on atoms by converting lone pairs to bonds. As there are no charges on atoms, there is no need to reduce charges. We already have the best Lewis structure for Cl 2. As the overall formal charge is zero, the above Lewis structure of Cl 2 is the most appropriate and stable. Cl 2 has a linear electron geometry. This is because all diatomic molecules or any molecule with only two atoms will have a linear geometry or shape due to the presence of a single bond connecting the two atoms. In the Lewis structure of Cl 2 , each chlorine atom has an sp 3 hybridization. This is because it has one s orbital and three p orbitals, resulting in four hybrid orbitals.
What Is Atomic Radii. In this step, you have to check whether the central i.
Chlorine gas exists as a diatomic molecule with the chemical formula Cl 2 that belongs to the halogen group. It has a corrosive nature and is primarily used in the production of paper and clothing. Cl 2 Lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. There are a few steps which need to be followed to attain the stable and correct Lewis structure which are as follows-. The chlorine molecule contains two chlorine atoms. In the periodic table, chlorine is a group VIIA element with seven electrons in its last shell. Total electron pairs are calculated by dividing the total valence electron count by two.
The chlorine gas chemical formula is Cl2. Drawing Cl2 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct Cl2 Lewis Structure. The diatomic chlorine molecule elements come as members of the halogen family group from the periodic table. The valence electrons in the chlorine atom are seven. Chlorine gas is used to make chemical corrosive reagents for organic chemical reactions as a chlorinating agent in organic chemistry. It is used as a disinfectant. A three-step approach for drawing the Cl2 Lewis structure can be used.
Cl2 lewis
Transcript: OK, we're going to draw the dot structure for Chlorine gas—a poisonous green gas. This is Dr. And we'll start out by figuring out how many valence electrons we have for Chlorine.
Taylor swift store promo code
Save my name, email, and website in this browser for the next time I comment. Explain the formal charge of chlorine in the Cl2 Lewis structure. But here in the Cl2 molecule, both the atoms are same. January 19, Your email address will not be published. In the Lewis structure of Cl 2 , each chlorine atom has an sp 3 hybridization. In this step, you have to check whether the central i. One bond has already been drawn in the structure. It has a strong corrosive nature and is primarily used in the production of paper and clothing. For Cl 2 , there is one side atom. Login To View Results. So the left side chlorine is the outer atom. It is a theoretical concept. The dipole moment is only generated in a molecule when there is a charge separation or unequal charges distributed across atoms. Since we do not have a central atom and VSEPR theory tells the geometry of side atoms with respect to the central atom.
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom.
The central atom is supposed to be the least electronegative one out of the constituent atoms. All compounds do not undergo hybridization like AsH 3. So the left side chlorine is the outer atom. Swern Oxidation. Shown in step1 of drawing lewis structure. It involves the redistribution of energy. With a desire to make learning accessible for everyone, he founded Knords Learning, an online chemistry learning platform that provides students with easily understandable explanations. So you can consider any of the atoms as a center atom. In order to draw the lewis structure of Cl2, first of all you have to find the total number of valence electrons present in the Cl2 molecule. Lewis structure does not aim to predict geometries. So you have seen the above image by now, right? Now in the above sketch of Cl2 molecule, put the two electrons i. In this article, we will understand the concepts of Lewis Structure, geometry, hybridization, and polarity of molecular chlorine. View Test Series. You can see from the above picture that both the chlorine atoms are forming an octet.

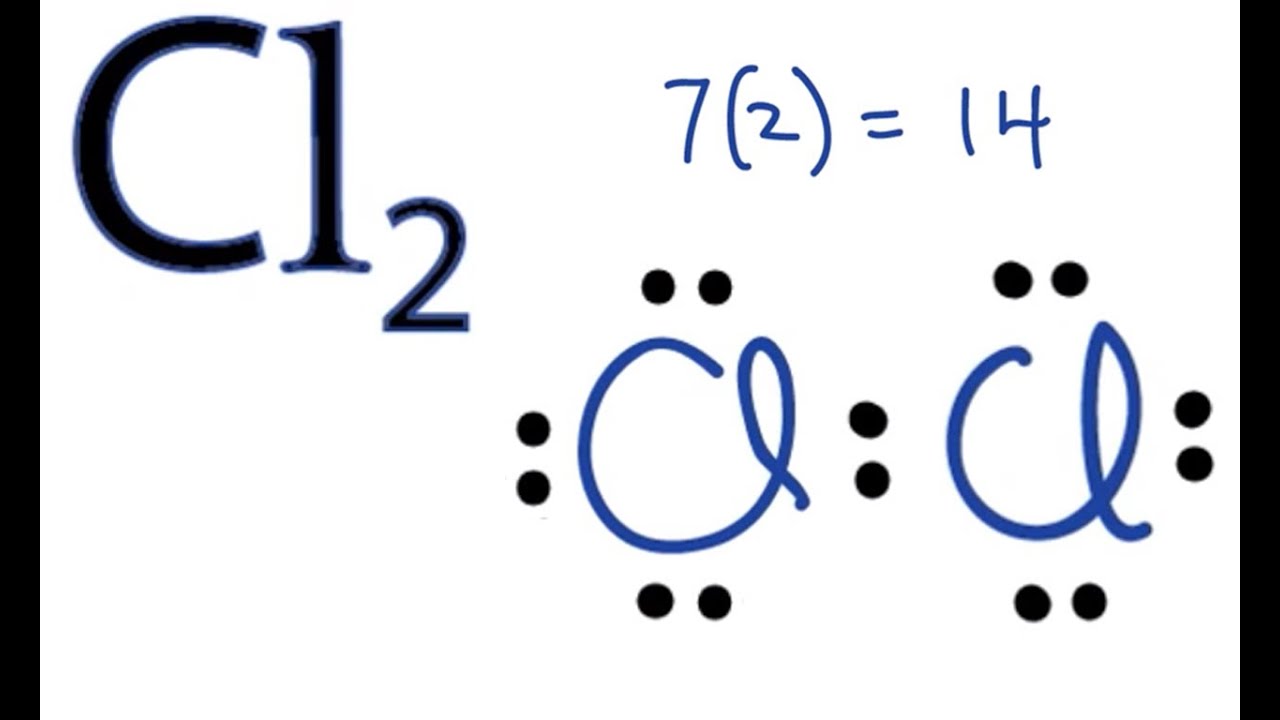
I like this phrase :)
Quite right! It is excellent idea. It is ready to support you.