Cyanate lewis structure
Ready to learn how to draw the lewis structure of OCN- ion cyanate ion? Here, I have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images.
There is a -1 formal charge on the Oxygen atom O. In order to find the total valence electrons in an OCN- cyanate ion ion, first of all you should know the valence electrons present in oxygen atom , carbon atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Oxygen is group 16 element on the periodic table. Carbon is group 14 element on the periodic table. Nitrogen is a group 15 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Cyanate lewis structure
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca. This high frequency resulted in the conclusion that this bond was a triple bond. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons. Either of the lone pairs can be accepted by Lewis acceptors. Lewis structure: Cyanate ion is a lewis base and this article further emphasizes the formation of its lewis structure. Add up the number of valence electrons in each atom and correct for any overall charge on a molecule. Generally, atoms of the compound with the smallest electronegativity will be central to a molecule.
We're still only using 16 valence electrons. So it does not fulfill the octet rule. According to the above facts and the given table, we can say that cyanate lewis structure geometry of cyanate ions will be linear and it will be sp hybridized.
That includes this negative up here. Carbon is the least electronegative; we'll put that at the center. Then an Oxygen here, and a Nitrogen over here. We'll put 2 electrons between atoms to form a chemical bond. Then we'll go around the outside, so we have 2, 4, 6, 8, 10, 12, 14,
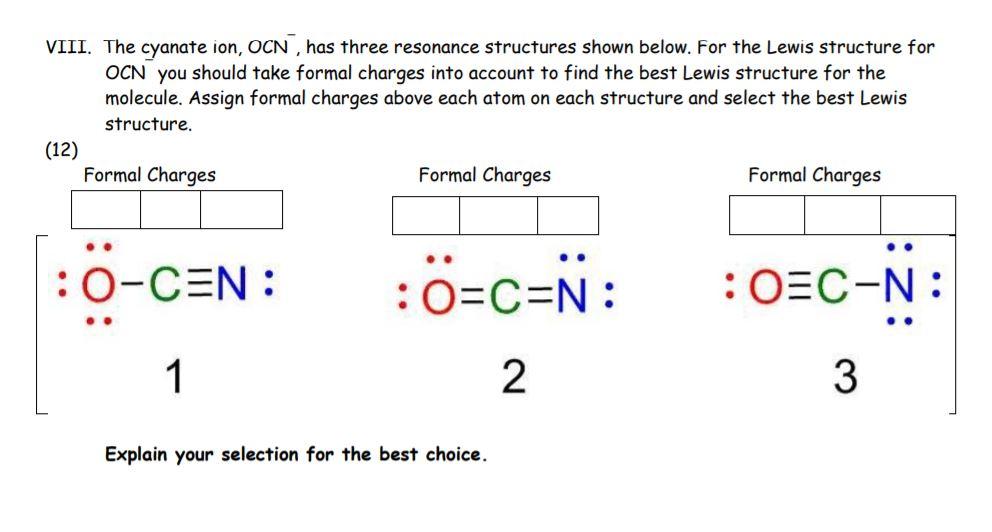
The cyanate ion is an anion composed of one oxygen atom, one carbon atom, and one nitrogen atom, in the order [OCN]. It possesses 1 unit of a negative charge, borne by the nitrogen atom. It is an ambident nucleophile in nucleophilic substitution. It is relatively non-toxic in comparison with cyanides. We will now learn about the Lewis structure of this molecule to understand its physical and chemical properties. The structure will help better understand the arrangement of atoms, bond formation and shape of the molecule.
Cyanate lewis structure
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors.
Null meaning in punjabi
He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Valence electrons are the number of electrons present in the outermost shell of an atom. The cyanate ion works as an ambidentate ligand. Now the question is, from which atom should we move the electron pair? Oxygen is group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. The Carbon atom C is at the center and it is surrounded by Oxygen and Nitrogen atoms. So again we have to shift one more electron pair from the nitrogen atom only. So let's move them from the Nitrogen. Now, you have come to the final step and here you have to check the formal charge on Oxygen atom O , Carbon atom C as well as Nitrogen atom N. So the above lewis dot structure of OCN- ion can also be represented as shown below. Well, we should move the electron from the atom which is less electronegative. By doing so, you will get the following lewis structure of OCN- ion.
Any salt containing the ion, such as ammonium cyanate , is called a cyanate.
Also, in step 1 we have calculated the total number of valence electrons present in the OCN- ion. Step 2 : Either it can share two bonds or carbon can form a triple bond with nitrogen and oxygen. Oxygen is group 16 element on the periodic table. As you can see from the above image, the central atom i. Here, nitrogen is less electronegative than oxygen. The Polarity of a Cyanate ion. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Unfortunately, the carbon atom is not forming an octet here. Now the question is, from which atom should we move the electron pair? Cyanate usually yields an isocyanate in nucleophilic substitution reactions. So now, you have to complete the octet on these outer atoms. The shape of a molecule can be determined by the number of electrons in its atoms and how the electrons repel each other. Here, the given ion is OCN- cyanate ion. You'd see that that would work from an octet standpoint, but when we check our formal charges, we'd have a -1 on the Nitrogen.

At someone alphabetic алексия)))))