Define water of crystallization class 10
The chemical formulae of two compounds can be same. Do you agree? Water of crystallisation in compound P:. What is meant by water of crystallisation in a substance?
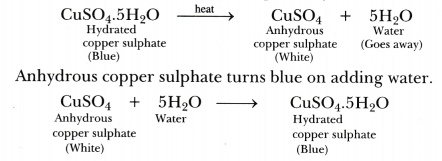
Last updated at May 29, by Teachoo. Copper Sulphate CuSO 4 is ideally white and powdery and does not have any water anhydrous. When we add water to these crystals they turn blue due to the formation of Copper pentahydrate CuSO 4. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Your browser does not support the audio element. Maths Classes.
Define water of crystallization class 10
Crystallization is a technique for separating solids from a solution or, to put it another way, a procedure for purifying things. This is the most frequent method for purifying seawater. Some salts have a few water molecules in their crystal structure as an essential component. Hydrated salts are salts that contain the water that causes crystallization. Below is a detailed explanation of crystallisation of water, hydrated and anhydrous salts, and also the action of heat on the hydrated salts. The water of crystallization refers to the water molecules that make up the structure of a salt crystal. Water that has been chemically linked into a crystal structure is known as the water of crystallization. The production of crystals frequently requires the use of water. A fixed number of molecules in one formula of a unit of salt is referred to as water of crystallization. Hydrates are crystal salts that contain water during the crystallization process. The water of crystallization is also known as the water of hydration or crystallization water.
Some salts have a few water molecules in their crystal structure as an essential component. Old search 2. View More.
In chemistry, water s of crystallization or water s of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt , which is not directly bonded to the metal cation. Upon crystallization from water, or water-containing solvents , many compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. Compared to inorganic salts , proteins crystallize with large amounts of water in the crystal lattice. Knowledge of hydration is essential for calculating the masses for many compounds.
Crystallization is a technique for separating solids from a solution or, to put it another way, a procedure for purifying things. This is the most frequent method for purifying seawater. Some salts have a few water molecules in their crystal structure as an essential component. Hydrated salts are salts that contain the water that causes crystallization. Below is a detailed explanation of crystallisation of water, hydrated and anhydrous salts, and also the action of heat on the hydrated salts. The water of crystallization refers to the water molecules that make up the structure of a salt crystal.
Define water of crystallization class 10
Question 1 What is meant by water of crystallisation? Give example? Question 2 What are hydrated salts?
How a highwayman might confess
The water of crystallisation is the total quantity of water in a substance at a certain temperature and is usually present in a definite ratio in different situations. It losses water molecules and a white powder C u S O 4 is obtained. Work Experiences. This is the most frequent method for purifying seawater. Facebook Whatsapp. Morosin Water of Crystallization. Tools Tools. Define water of crystallisation. What is the role of pH in our Digestive System? In the table below are indicated the number of molecules of water per metal in various salts. The production of crystals frequently requires the use of water. Hi, it looks like you're using AdBlock :.
Define water of crystallization? Give the chemical formula for two compounds as examples? How can it be proved that the water of crystallization makes a difference in the state and color of the compounds?
Teachoo gives you a better experience when you're logged in. Water is particularly common solvent to be found in crystals because it is small and polar. Pt-Pt bonded Chinese lantern structure [34]. Copper is surrounded by six oxygen atoms, provided by two different sulfate groups and four molecules of water. Interview Experiences. When copper sulphate crystals are heated to a high temperature, they lose all of their water and become anhydrous copper sulphate, which is white. A person may suffer from both myopia and hypermetropia defects. An unknown sample can be determined through thermogravimetric analysis TGA where the sample is heated strongly, and the accurate weight of a sample is plotted against the temperature. Question 5: How is the water of crystallization useful for the crystals of salts? One of several nickel sulfate hydrates [33]. A concave mirror is used for image formation for different positions o Share your suggestions to enhance the article. Suggest Changes. The presence of moisture or water in anhydrous copper sulphate is indicated by the appearance of blue colour.

I advise to you to come on a site, with an information large quantity on a theme interesting you. There you by all means will find all.
Quite right! It is excellent idea. I support you.