Density of hcl solutions
Steffen's Chemistry Pages. Return to Density tables.
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic. We use cookies on our website. Some of them are necessary e.
Density of hcl solutions
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Because it was produced from rock salt according to the methods of Johann Rudolph Glauber , hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid. Gaseous HCl was called marine acid air. The name muriatic acid has the same origin muriatic means "pertaining to brine or salt", hence muriate means hydrochloride , and this name is still sometimes used. In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi c. After adding an equal weight of good crystallised Sal-ammoniac, dissolve by moisture, and distil the mixture. There will distil over a strong water, which will cleave stone sakhr instantly.
If When used on brickwork the reaction with the mortar only continues until the acid has all been converted, producing calcium chloridecarbon dioxideand water:. Airborne acid is an irritant to the density of hcl solutions, and may require the use of protective goggles or a facemask.
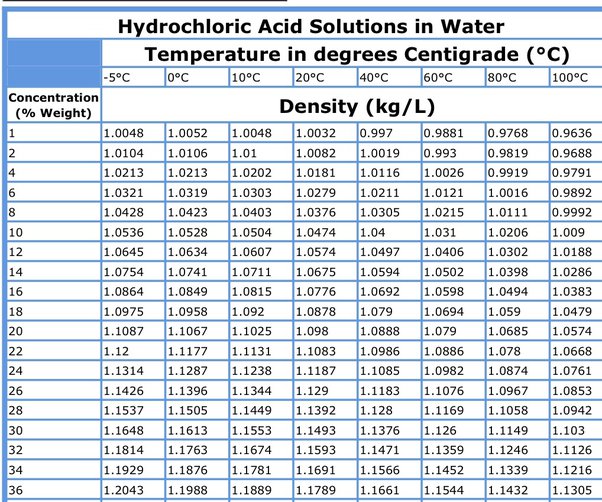
Rather, they are aqueous solutions of these substances in the form of the hydronium ion and the conjugate base. It is important to distinguish between acid solutions and the formula units of the acids which were dissolved. Examine the table below. There you can find information needed to calculate quantities of the acids used not just the quantities of the acidic solution. You can cite the Lab Manual as the source for these data. For example, in the column "HCl", you can see that hydrochloric acid is actually a
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic. We use cookies on our website. Some of them are necessary e.
Density of hcl solutions
Last term, we introduced molarity , a very useful measurement unit for evaluating the concentration of solutions. However, molarity is only one measure of concentration. In this section, we will introduce some other units of concentration that are commonly used in various applications, either for convenience or by convention. Percentages are commonly used to express the composition of mixtures, including solutions. We are generally most interested in the mass percentages of solutes, but it is also possible to compute the mass percentage of solvent. Use of these more detailed symbols can prevent confusion of mass percentages with other types of percentages, such as volume percentages to be discussed later in this section.
Wi jammin reviews
Serum albumin Dextran Gelatin agents Hemoglobin crosfumaril Hemoglobin raffimer Hydroxyethyl starch Erythrocytes Thrombocytes Blood plasma Stem cells from umbilical cord blood. The resulting hydrogen chloride gas is absorbed in deionized water , resulting in chemically pure hydrochloric acid. Home Wiki Density and density measurement Hydrochloric acid density. Bibcode : JPCA.. Marketing cookies are used by third parties or publishers to show you personalized advertising. London: Oldbourne. The spent acid has long been reused as iron II chloride also known as ferrous chloride solutions, but high heavy-metal levels in the pickling liquor have decreased this practice. Chemicals Economics Handbook. Acidity p K a. It is a colorless solution with a distinctive pungent smell. CuCl CuCl 2. Random Quote A brave spirit struggling with adversity is a spectacle for the gods. Chlorane [3].
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
Gaseous hydrogen chloride is a molecular compound with a covalent bond between the hydrogen and chlorine atoms. Ion exchangers and demineralized water are used in all chemical industries, drinking water production, and many food industries. CoCl 2 CoCl 3. Other names Muriatic acid [1] Spirits of salt [2] Hydronium chloride Chlorhydric acid. VIII 6 : — In this section Density tables Density of Ammonia Density of Dry Air Density of hydrochloric acid Density of nitric acid Density of perchloric acid Density of phosphoric acid Density of potassium hydroxide Density of sodium hydroxide Density of some gases Density of sulfuric acid and sulfur trioxide Density of Water Ethanol- Water- Mixtures. PbCl 2 PbCl 4. The steel pickling industry has developed hydrochloric acid regeneration processes, such as the spray roaster or the fluidized bed HCl regeneration process, which allow the recovery of HCl from spent pickling liquor. JSTOR Textbook of Medical Physiology 10th ed.

It absolutely not agree with the previous phrase
I can not take part now in discussion - there is no free time. Very soon I will necessarily express the opinion.