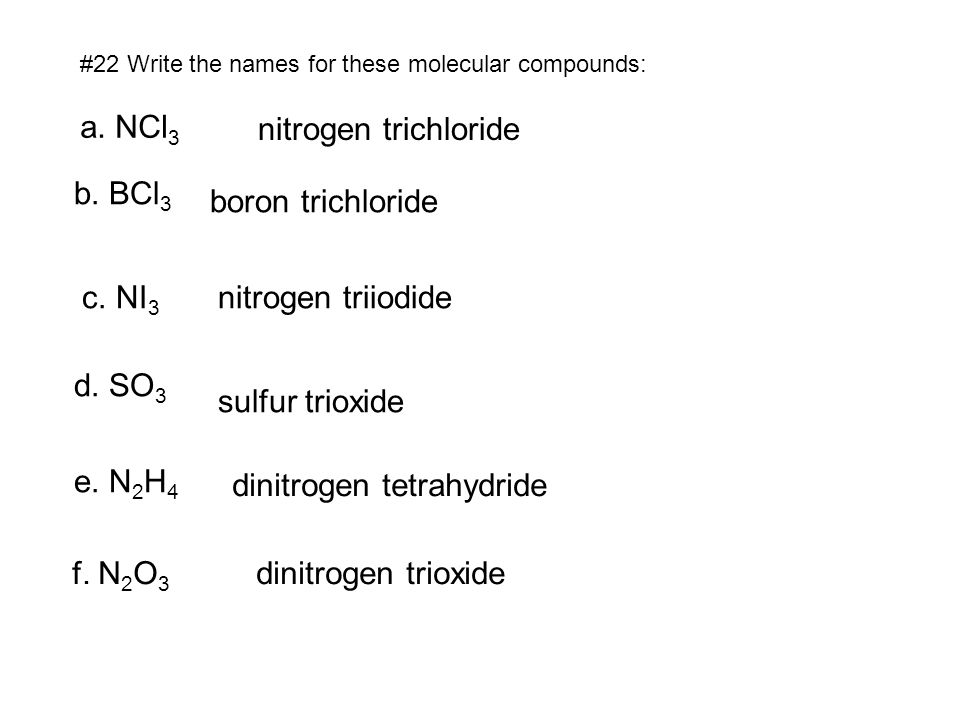
Dinitrogen tetrahydride
Submitted by Parker P.
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Myron Cummings Modified over 8 years ago.
Dinitrogen tetrahydride
E-mail: ynishiba sogo. A dinitrogen-bridged dimolybdenum-tetrachloride complex is prepared and reduced with Super-Hydride LiBHEt 3 to afford the corresponding dimolybdenum-dinitrogen complex together with the formation of molecular dihydrogen. This reaction proceeds via the ligand exchange of the coordinated dihydrogen generated in situ with molecular dinitrogen. As the next stage of the previous work, we have focused on the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen at ambient temperature and pressure. To achieve the catalytic formation of ammonia as the next nitrogen fixation, in place of the Haber—Bosch process, 7 the ruthenium—hydride species should reduce the high oxidative tungsten species to regenerate the corresponding tungsten—dinitrogen complex. However, unfortunately, the tungsten species can not be reduced with the ruthenium—hydride species or with other hydride species such as LiBHEt 3. As the first stage of the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen under mild reaction conditions, we envisaged the reaction of the high oxidative molybdenum complexes with hydride species to regenerate the corresponding dinitrogen complexes as starting catalytic species. In this reaction, the high oxidative molybdenum complexes can be reduced into the corresponding dinitrogen complexes, where the ligand exchange of the coordinated dihydrogen with molecular dinitrogen may be involved as a key step to regenerate the corresponding dinitrogen complexes. Herein, we describe the preparation of the dinitrogen-bridged dimolybdenum-tetrachloride complex bearing a PNP -type pincer ligand and the reduction of the dimolybdenum-tetrachloride complex with Super-Hydride LiBHEt 3 to afford the corresponding molybdenum-dinitrogen complex together with the formation of molecular dihydrogen. No informative data on the structure of 2 were obtained from its NMR spectra due to the paramagnetism. A more detailed molecular structure of 2 is determined by X-ray crystallographic study Fig. The molecular structure of 2 contains two [MoCl 2 PNP ] moieties bearing two chloride ligands in trans form. The two molybdenum atoms are bridged by one dinitrogen ligand in an end-on fashion with the almost linear Mo—N—N—Mo bonding. The two molybdenum fragments are twisted around the Mo—N—N—Mo axis with respect to each other away from the steric interaction between two PNP ligands: the torsion angle for Cl 1 —Mo 1 —Mo 2 —Cl 3 is
Find UBC Chemistry on.
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet. How it is incorporated into the biosphere is complicated by its intrinsic inertness. For example, biological nitrogen fixation takes N-2 and converts it into ammonia using various nitrogenase enzymes, whereas industrial nitrogen fixation converts N-2 and H-2 to NH3 using heterogeneous iron or ruthenium surfaces. In both cases, the processes are energy-intensive. Is it possible to discover a homogeneous catalyst that can convert molecular nitrogen into higher-value organonitrogen compounds using a less energy-intensive pathway? If this could be achieved, it would be considered a major breakthrough in this area. In contrast to carbon monoxide, which is reactive and an important feedstock in many homogeneous catalytic reactions, the ischelectronic but inert N-2 molecule is a very poor ligand and not a common industrial feedstock, except for the above-mentioned industrial production of NH3.
This chapter describes the activation of dinitrogen by various transition metal hydride complexes. A number of mononuclear transition metal hydride complexes can incorporate dinitrogen, but they are usually difficult to induce N—N bond cleavage. In contrast, multimetallic hydride complexes can split and hydrogenate dinitrogen through cooperation of the multiple metal hydrides. In this transformation, the hydride ligands serve as the source of both electron and proton, thus enabling the cleavage and hydrogenation of dinitrogen without extra reducing agents and proton sources. This is a preview of subscription content, log in via an institution. Luo YR Comprehensive handbook of chemical bond energies. Book Google Scholar.
Dinitrogen tetrahydride
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms the elements of group 14 are carbon , silicon , germanium , tin , lead and flerovium. The tetrahydride series has the chemical formula XH 4 , with X representing any of the carbon family. Methane is commonly the result of the decomposition of organic matter and is a greenhouse gas. The other hydrides are generally unstable, poisonous metal hydrides. They take on a pyramidal structure, and as such are not polar molecules like the other p-block hydrides. Unlike other light hydrides such as ammonia , water and hydrogen fluoride , methane does not exhibit any anomalous effects attributed to hydrogen bonding , and so its properties conform well to the prevailing trend of heavier group 14 hydrides. This series has the chemical formula X 2 H 6.
Ipod nano button stuck
One crew member lost consciousness during descent. It is also the primary oxidizer for Russia's Proton rocket. Login Sign up. How this fails for each particular reagent is discussed and evaluated. We'll give when reacted with N Freeman, p. Toggle navigation Faculty of Science Department of Chemistry. One of the earliest uses of this combination was on the Titan family of rockets used originally as ICBMs and then as launch vehicles for many spacecraft. Dinitrogen tetrahydride reacts with dinitrogen tetraoxide to form nitrogen gas and water. J: Prentice Hall. Freeman, p.
Hydrazine is a molecule of two singly-bonded nitrogen atoms and four peripheral hydrogen atoms.
How this fails for each particular reagent is discussed and evaluated. J: Prentice Hall. What is covalent bonding? Identify the atoms that have been oxidized and reduced, and identify the oxidizing and reducing agents. PubChem CID. Hydrogen compounds are handled differently and will be looked at first. Such dissociative gas Brayton cycles have the potential to considerably increase efficiencies of power conversion equipment. Toggle limited content width. The tendency of N 2 O 4 to reversibly break into NO 2 has led to research into its use in advanced power generation systems as a so-called dissociating gas. In early , research on the usability of dinitrogen tetroxide as an oxidizing agent for rocket fuel was conducted by German scientists, although the Germans only used it to a very limited extent as an additive for S-Stoff fuming nitric acid. Nitric acid is manufactured on a large scale via N 2 O 4. The answer attempts to explain the balanced chemical equation, but the presentation of the solution is confusing and could use better organization. It is a hypergolic propellant in combination with a hydrazine -based rocket fuel. Chapter 4 Compounds and Their Bonds 4.

Absolutely with you it agree. It is good idea. I support you.
I consider, that you commit an error. I suggest it to discuss. Write to me in PM, we will communicate.
It agree, rather useful idea