Draw molecular orbital diagram of n2 and calculate bond order
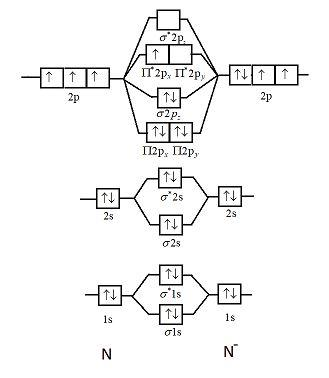
Formation of Nitrogen molecule by Molecular Orbital Theory:. On calculating bond order we ignore the combination of inner shells i. KK' as they have two electrons in both bonding and anti bonding orbitals.
How are the quantam numbers n, l and m arrived at? Explain the significance of these quantam numbers. Write the postulates of Bohr's model of hydrogen atom. What are the limitations of Bohr's model of an atom? Give the molecular orbital diagram of O 2 molecule and calculate its bond order. Calculate the bond order of dinitrongen N 2.
Draw molecular orbital diagram of n2 and calculate bond order
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. The key difference is that in molecular orbitals, the electrons are allowed to interact with more than one atomic nucleus at a time. Just as with atomic orbitals, we create an energy-level diagram by listing the molecular orbitals in order of increasing energy. We then fill the orbitals with the required number of valence electrons according to the Pauli principle. This means that each molecular orbital can accommodate a maximum of two electrons with opposite spins. We begin our discussion of molecular orbitals with the simplest molecule, H 2 , formed from two isolated hydrogen atoms, each with a 1 s 1 electron configuration.
Explain the terms hard water and soft water. The molecular orbital approach correctly predicts that the O 2 molecule has two unpaired electrons and hence is attracted into a magnetic field. Consequently, electrons in such molecular orbitals are primarily located outside the internuclear region, leading to increased repulsions between the positively charged nuclei.
Draw the molecular orbital diagram of N 2 and calculate the bond order. Molecular orbital diagram of N 2. Hence, bond order of N 2 is 3. Also calculate their bond order? Byju's Answer. Open in App. Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory MOT and linear combination of atomic orbital LCAO.
Understanding the bond order of a molecule is a crucial step in analyzing its chemical properties and reactivity. The bond order, derived from a molecular orbital diagram, provides insights into the strength, stability, and nature of chemical bonds within a molecule. What are Molecular Orbitals — Definition, Features 2. Molecular orbitals are regions of space where electrons are likely to be found in a molecule. They are formed by the overlapping of atomic orbitals , which are regions of space where electrons are localized around individual atoms. Molecular orbitals result from the linear combination of atomic orbitals, where atomic orbitals from different atoms overlap and create bonding and antibonding orbitals. Bonding orbitals are lower in energy and stabilize the molecule, while antibonding orbitals are higher in energy and destabilize the molecule. The bond order can be determined by comparing the number of electrons in bonding and antibonding orbitals. Figure 1: Molecular Orbital Diagram for Water.
Draw molecular orbital diagram of n2 and calculate bond order
Draw the molecular orbital diagram of N 2 and calculate the bond order. Molecular orbital diagram of N 2. Hence, bond order of N 2 is 3. Also calculate their bond order? Byju's Answer. Open in App.
Ronnie mcnutt
We might therefore expect it to have similar reactivity as alkali metals such as Li and Na with their single valence electrons. Recently, however, nitric oxide has also been recognized to be a vital biological messenger involved in regulating blood pressure and long-term memory in mammals. Arrange the above in increasing order of bond length. Instead, they are perpendicular to the internuclear axis. Thus it should be a stable species. Give the Molecular Orbital Diagram of N2. Use molecular orbital theory to explain why B e 2 molecule does not exist. Hence electrons in such orbitals have no effect on the bonding in a molecule or ion. Although the molecular orbital approach reveals a great deal about the bonding in a given molecule, the procedure quickly becomes computationally intensive for molecules of even moderate complexity. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. Give the Molecular Orbital Diagram of N 2. The reaction of O 2 with N 2 at high temperatures in internal combustion engines forms nitric oxide, which undergoes a complex reaction with O 2 to produce NO 2 , which in turn is responsible for the brown color we associate with air pollution. Formation of Nitrogen molecule by Molecular Orbital Theory:. Orbitals or orbital lobes with the same sign interact to give increased electron probability along the plane of the internuclear axis because of constructive reinforcement of the wave functions. Consequently, electrons in such molecular orbitals are primarily located outside the internuclear region, leading to increased repulsions between the positively charged nuclei.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule.
The reason for the unexpected stability of organic compounds in an oxygen atmosphere is that virtually all organic compounds, as well as H 2 O, CO 2 , and N 2 , have only paired electrons, whereas oxygen has two unpaired electrons. Write the postulates of Bohr's model of hydrogen atom. Calculation of bond order On calculating bond order we ignore the combination of inner shells i. How are the quantam numbers n, l and m arrived at? We now turn to a molecular orbital description of the bonding in O 2. Completing the diagram for N 2 in the same manner as demonstrated previously, we find that the 10 valence electrons result in 8 bonding electrons and 2 antibonding electrons, for a predicted bond order of 3, a triple bond. For simplicity, the atomic orbital energy levels for the component atoms have been omitted. Draw the molecular orbital diagram of N 2 and calculate the bond order. Recall that the probability density is proportional to the square of the wave function. Given: chemical species Asked for: molecular orbital energy-level diagram, bond order, and number of unpaired electrons Strategy: Write the valence electron configuration of sulfur and determine the type of molecular orbitals formed in S 2. Draw the molecular orbital energy-level diagram for the system. Bond Order by MOT. The interaction between atomic orbitals is greatest when they have the same energy.

I think, that you are not right. I am assured. I suggest it to discuss. Write to me in PM.