Einstein photoelectric effect class 12
Read till the end to know more. In this article, we will understand how Einstein explained the photoelectric effect and why earlier scientists failed symbollab do this.
The photoelectric effect is a phenomenon where electrons are emitted from the metal surface when light of sufficient frequency is incident upon it. The concept of the photoelectric effect was first documented in by Heinrich Hertz and later by Lenard in Hertz who had proved the wave theory himself did not pursue the matter as he felt sure that it could be explained by the wave theory. However, the concept failed in the following accounts:. Electrons emitted from underneath the metal surface lose some kinetic energy during the collision.
Einstein photoelectric effect class 12
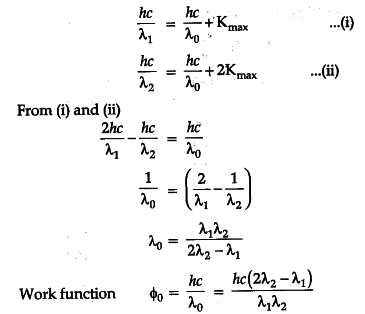
Einsteins photoelectric equation is. Write Einstein's photoelectric equation. What is photoelectric effect? Mention the Einstein's photoelectric equation. Explain the laws of photoelectric effect from the Einstein photoelectric equation. By photoelectric effect, Einstein, proved. Define the threshold frequency v 0 and stopping potential V 0. Write Einsteins's photoelectric equation. Explain the terms i threshold frequency, and ii stopping potential. Einstein's work on photoelectric effect gives support to. What is the photoelectric effect? Frame Einstein. The threshold frequency of a photoelectric effect depends on.
According to the Photoelectric effect, when a metal surface is irradiated with light of sufficient energy, it causes the electrons of the metal to eject out.
Albert Einstein, a German physicist, is considered one of the greatest scientists of all times. He has contributed to the fields of general relativity , black holes, photoelectric effect, to name a few. In , Einstein was awarded the Nobel Prize in physics for the discovery of the photoelectric effect. Einstein's idea about light was revolutionary and magnificent. He gave an efficient method of irradiation. Light has some tiny group of particles known as photons. These particles consist of higher energy, which is also called the quantum of radiation.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Einstein's Photoelectric Equation. Explaining the experiments on the photoelectric effect. How these experiments led to the idea of light behaving as a particle of energy called a photon. Key points. Based on the wave model of light, physicists predicted that increasing light amplitude would increase the kinetic energy of emitted photoelectrons, while increasing the frequency would increase measured current.
Einstein photoelectric effect class 12
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Why light and matter are two faced!
Amazon lr44 batteries
What is threshold frequency in the photoelectric effect? Access more than. It is independent of the intensity of incident radiation. Learn more. The distribution of electronic conditions in terms of strength and pressure — the structure of the solid electronic band — determines the electronic characteristics of the ordered, shiny solid materials. Login To View Results. It will help you understand the depths of this important device and help solve relevant questions. It is named after Thomas Young. These particles consist of higher energy, which is also called the quantum of radiation. The values obtained by this equation are in congruence with actual values, thus proving Einstein's explanation for the Photoelectric effect. Using this, we have. This is called the wave-particle duality of light. In , Einstein was awarded the Nobel Prize in physics for the discovery of the photoelectric effect.
In , the annus mirabilis miracle year of Physics, Albert Einstein proposed an equation to explain this effect. Einstein argued that light was a wave that interacts with matter in the form of a packet of energy or a quantum of energy.
What are the applications of photoelectric effect? Learn more topics related to Physics. This meant that the kinetic energy of electrons would increase with that of light intensity. Young's modulus is a measure of the elasticity or extension of a material when it's in the form of a stress—strain diagram. Hence, the work function of a metal is directly related to the threshold frequency v0. Define the threshold frequency v 0 and stopping potential V 0. These effects can be explained by two assumptions, according to Einstein: light is made up of corpuscles or photons, whose power is determined by Planck's equation, and a single metal atom can absorb a whole photon or anything. The energy photon is hv , where h is constant and v is the frequency of light. Incident radiation of higher frequency makes the electrons vibrate faster, thereby increasing the electron emission. Experimental physicists faced a major challenge in the late 's. Hence initially, the photoelectric effect was discovered by Hertz and Lenard; it could not do justice to the equation given by Planck. This is the photoelectric effect.

I congratulate, remarkable idea and it is duly
In my opinion you commit an error. Let's discuss. Write to me in PM.