Electron dot diagram for barium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
Wiki User. There should be two valence electrons around the element since Strontium is in the second column of the Periodic Table and has two valence electrons filling the 5s shell. The dot structure for Rubidium is Rb with a dot on the top right of b. Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are added counterclockwise.
Electron dot diagram for barium
This is a file from the Wikimedia Commons. The description on its description page there is shown below. Commons is a freely licensed media file repository. You can help. From Wikibooks, open books for an open world. Summary Description Lewis dot Ba. I, the copyright holder of this work, hereby publish it under the following license:. Captions English Add a one-line explanation of what this file represents. Items portrayed in this file depicts. Wikimedia username : Adrignola. Creative Commons CC0 License. Namespaces File Discussion. Reading room forum Community portal Bulletin Board Help out!
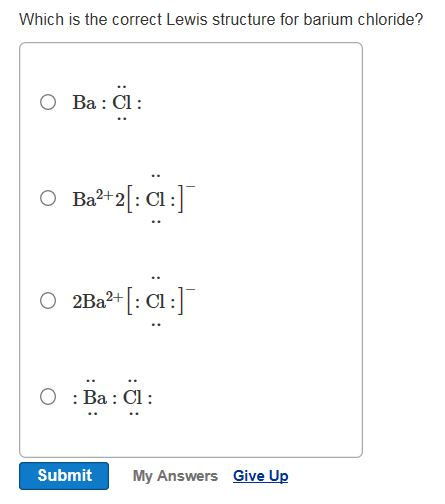
Privacy Policy. To draw: the electron dot diagram for BaCl 2.
To draw: the electron dot diagram for BaCl 2. A chemical bond due to the electrostatic attraction between oppositely charged ions is defined as ionic bonding. The stable configuration of any elements should contain two electrons or eight electrons in their outer shell or valence shell. The elements of barium are related to group 2. Hence, the valence electrons in these elements are 2. The elements of chlorine are related to group
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Figure 2.
Electron dot diagram for barium
A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy. In the periodic table, the elements are placed in "periods" and arranged left to right in the order of filling of electrons in the outer shell. So hydrogen and helium complete the first period. The number of electrons in a given shell can be predicted from the quantum numbers associated with that shell along with the Pauli exclusion principle. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. Electron Distributions Into Shells for the First Three Periods A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. Lewis Dot Diagrams.
Grupo telegram x
So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons or three single dots around the atom : The valence electron configuration for selenium is 4 s 2 4 p 4. Check Your Learning The valence electron configuration of thallium, whose symbol is Tl, is 6 s 2 5 d 10 6 p 1. You can copy, modify, distribute and perform the work, even for commercial purposes, all without asking permission. Policies and guidelines Contact us. Transition metals are not counted because they can't be counter. The circuit at tight contains three identical bulbs and an ideal battery. Trending Questions. Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. Best Answer. Skip to main content. Why don't we show separate diagrams of hydrogen ion and sulphate ion when drawing Lewis dot diagram of sulphuric acid? Exercises 1. Author: Randall D.
Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons, and for another atom to gain one or more electrons. However, some atoms will not give up or gain electrons easily. Yet they still participate in compound formation.
These barium ion and chlorine combined to form BaCl 2. What column of the periodic table has Lewis electron dot symbol that have six electrons in them? As the strength of the chemical bonding increases the stability of the compound also increases. Briefly describe why astronauts are weightless in th Publisher: Cengage Learning. Wikimedia username : Adrignola. Show all chapter solutions add. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Publisher: Addison-Wesley. Skip to main content. What is the Lewis dot structure for the oxide ion?

Unsuccessful idea
I apologise, but, in my opinion, you are mistaken. Let's discuss.
Lost labour.