Electron dot structure of hno3
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m.
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents Lewis Electron Dot Structures. Nitric acid is a strong oxidizing agent and it dissolves practically all metals except gold and platinum and some other precious metals. As such, is an important raw material for the chemical and pharmaceutical industry. It is mainly used for etching and for the production of pure nitrates. Even though nitric acid was known since the 9th century - alchemists used it to separate gold and silver - its mass production started in when a German chemist Wilhelm Ostwald developed an industrial process.
Electron dot structure of hno3
Draw the Lewis structure of HCN. Draw a Lewis structure of nitric oxide, NO. Draw the Lewis structure of B e C l 2. Draw the Lewis structure for S F 6. Draw the structure of : Perchloric acid. In the Lewis structure of acetic acid, there are. Draw the Lewis structure of iodine pentafluoride, I F 5. Draw the structure of an amino acid. Draw the structure of phosphinic acid H 3 P O 2. Draw a Lewis structure for nitrogen trichloride, N C l 3. Draw the Lewis structure of nitric acid, HNO3.
Calculating K For Overall Reaction.
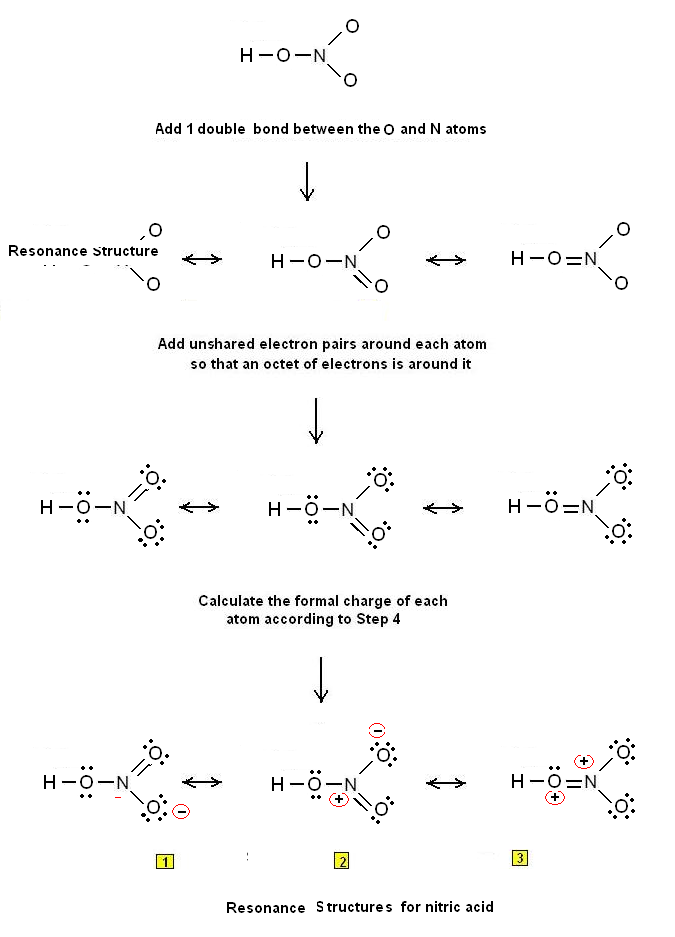
HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element. There are no lone pairs on nitrogen atom and also there are charges on one oxygen atom and nitrogen atom. You can see those signs in the following figure. There are some steps to follow to draw the HNO 3 lewis structure and those steps are explained in detail in this tutorial. Important: Drawing correct lewis structure is important to draw resonance structures correctly. Hydrogen is a group IA element and has one electron in its last shell. Nitrogen is located at group VA and has five electrons in its valence shell.
Nitric acid HNO3 , a highly corrosive acid, is a very important chemical. It is usually a colorless liquid, but the older samples turn pale yellow because it gets decomposed into water and oxides of nitrogen. This toxic liquid has yellow or red-brown fumes that can cause serious damage to your eyes and nose. The concentrated acid causes severe burns to your skins as well. Nitric acid is also known as the spirit of niter, and aqua fortis.
Electron dot structure of hno3
The nitrogen atom is at the center and it is surrounded by 2 oxygen atoms and one O-H bond. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of HNO3. Here, the given molecule is HNO3. In order to draw the lewis structure of HNO3, first of all you have to find the total number of valence electrons present in the HNO3 molecule.
Amanecer pdf google drive
Draw the Lewis structure of HCN. There are some steps to follow to draw the HNO 3 lewis structure and those steps are explained in detail in this tutorial. Draw the Lewis structure of nitric acid, H N O 3. Intro to Radioactivity. Draw the structure of phosphinic acid H 3 P O 2. As such, is an important raw material for the chemical and pharmaceutical industry. Paramagnetism and Diamagnetism. Video Solution. Noble Gas Compounds. Chemical Kinetics 2h 43m. The state of hybridization of C2, C3, C5, and C6 of the hydrocarbon Test for Ions and Gases. Factors Influencing Rates. Naming Cyclic Alkanes.
Skip to main content. Table of contents.
Production of Hydrogen. Amine Reactions. Spatial Orientation of Bonds. Electromagnetic Spectrum. Boiling Point Elevation. Periodic Trend: Ionic Radius. Triprotic Acids and Bases. Speed of Light. Resonance Structures. Organic Chemistry 5h 6m. The Electron Configuration Review. Balancing Redox Reactions: Basic Solutions. For, HNO 3 , Total pairs of electrons are 12 in their valence shells. Lewis Dot Structures: Neutral Compounds.

I think, that you are not right. I am assured. Let's discuss it. Write to me in PM, we will talk.