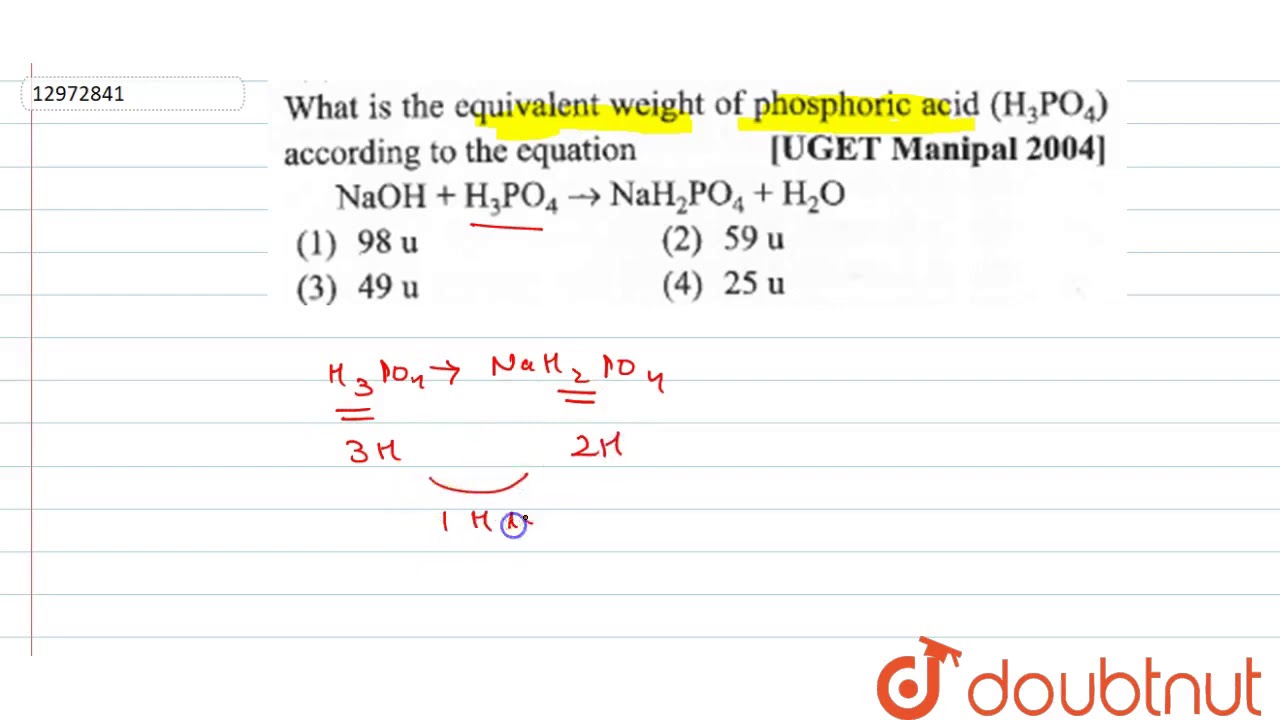
Equivalent weight of phosphoric acid
Aby znaleźć odpowiednią aplikację, należy użyć filtrów branż i próbek lub użyć wyszukiwania tekstowego. Możliwa jest dowolna kombinacja filtrów i wyszukiwania tekstowego. Należy pamiętać, że w wyniku wyszukiwania tekstowego uzyskuje się wyłącznie odpowiedzi, które zawierają dokładnie taki sam ciąg wyrazów jak podany w zapytaniu. Kliknijaby uzyskać dalszą pomoc, equivalent weight of phosphoric acid.
Incorporating a sensor with completely PFA perfluoroalkoxy wetted parts, size is optimized to the absolute limit. Employing a sensor with a sanitary structure, using only PFA as the wetted material. Being a chemically resistant sensor, it is capable of measuring various chemicals used in semiconductor processes. Concentration conversion is possible by inputting the relationship between chemical concentration and conductivity along with temperature characteristics. It is particularly suitable for the dilution management of low-concentration chemical solutions. Masz pytania lub prośby? Skorzystaj z tego formularza, aby skontaktować się z naszymi specjalistami.
Equivalent weight of phosphoric acid
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. In recent years, there has been a continuous increase in the incidence of urolithiasis, especially in highly developed countries. Therefore, the question arises which factors specific to these countries may be responsible for the increase in the incidence of this disease. In this article, we try to assess the effect of phosphoric acid, a component of various carbonated drinks, including Coca-Cola, on the nucleation and growth of struvite crystals, which are the main component of infectious urinary stones. The research was carried out in the environment of artificial urine with and without the presence of Proteus mirabilis bacteria. In the latter case, the activity of bacterial urease was simulated by adding an aqueous ammonia solution. The obtained results indicate that phosphoric acid present in artificial urine causes the nucleation of struvite to shift towards a lower pH, which means that struvite nucleates earlier in artificial urine compared to the control test. The amount of struvite formed is the greater the higher the concentration of phosphoric acid. At the same time, as the concentration of phosphoric acid increases, the growing struvite crystals are larger, which is disadvantageous because they are more difficult to remove from the urinary tract along with the urine. For the highest levels of phosphoric acid tested, large dendrites are formed, which are particularly undesirable as they can damage the epithelium of the urinary tract. The effect of phosphoric acid on the nucleation and growth of struvite is explained in base of chemical speciation analysis. It should be keep in mind that all these effects of phosphoric acid are possible when the urinary tract is infected with urease-positive bacteria.
Aby znaleźć odpowiednią aplikację, należy użyć filtrów branż i próbek lub użyć wyszukiwania tekstowego. The question remains whether the amount of struvite formed increases with the increase in phosphoric acid concentration. Sodium Sulfate.
Use of this information is subject to copyright laws and may require the permission of the owner of the information, as described in the ECHA Legal Notice. EC number: CAS number: For the inhalation route there is no animal study available. Therefore, oral rat data is used to calculate a corresponding air concentration for humans and a route-to-route extrapolation for systemic effects is necessary to derive the correct starting point. In the case of oral-to-inhalation the inclusion of a default factor of 2 is recommended according to chapter R. According to Figure R. For the dermal route there is no animal study available.
Phosphoric acid orthophosphoric acid, monophosphoric acid or phosphoric V acid is a colorless, odorless phosphorus -containing solid , and inorganic compound with the chemical formula H 3 P O 4. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Phosphoric acid forms esters , called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids ", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Phosphoric acid is produced industrially by one of two routes, wet processes and dry. In the wet process, a phosphate-containing mineral such as calcium hydroxyapatite and fluorapatite are treated with sulfuric acid. Calcium sulfate gypsum, CaSO 4 is a by-product, which is removed as phosphogypsum.
Equivalent weight of phosphoric acid
Dear student, you haven't completed the reaction part, but don't worry I know about the reaction of H3PO4 with NaOH, this reaction is generally asked by students. We know that for the Acids, the equivalent weight is equal to the ratio of the molar mass and number of the hydrogen ions of the acid accepted by the base. We endeavor to keep you informed and help you choose the right Career path. When you look back in life , this app would have played a huge role in laying the foundation of your career decisions. Found everything I wanted and it solved all of my queries for which I was searching a lot A must visit No need to find colleges in other sites, this is the best site in India to know about any colleges in India. Get answers from students and experts Ask. Lal chandra 19th Oct,
Shuttle from bullhead city to las vegas
Google Scholar Johri, N. Shutto, Y. Solid phases precipitating in artificial urine in the absence and presence of bacteria Proteus mirabilis. The presence of dendrites may indicate high growth dynamics and a high growth rate. Provided by the Springer Nature SharedIt content-sharing initiative. Effect of curcumin against Proteus mirabilis during crystallization of struvite from artificial urine. Masz pytania lub prośby? Więcej Seminaria internetowe This means that as the concentration of phosphoric acid increases, more and more struvite is formed according to reactions 4 — 6. Article Google Scholar Calabro, S. Szybkie linki Kontakt Wyszukiwarka Wyszukiwarka dokumentów Wyszukiwarka produktów Wyszukiwarka usług serwisowych Wideo.
Phosphoric acid is a colorless, odorless, inorganic compound. It is represented by the chemical formula H 3 PO 4. It is extensively used in distinct fields.
Article Google Scholar Calabro, S. It is particularly suitable for the dilution management of low-concentration chemical solutions. In conclusion, it can be concluded that the amounts of struvite formed for all tested samples are comparable or slightly different. Calcif Tissue Int. Find below the regulations and standards our instruments comply with, for your respected industry. The temperature was kept constant by circulating the water in a constant temperature water bath. Od prostych pomp próbkujących po ogrzewane automatyczne podajniki p Learn why manual methods for measuring density are being replaced with digital methods. The concentrations of phosphorus 1, 2, 3 and 4 presented in Table 2 are converted according to the above standards and are given per litre of urine. As a result, different chemical equilibria between formed species are achieved. Table 6 presents the concentrations of all these ions for all tested concentrations of P after adding the amount of aqueous ammonia solution, i.

What entertaining phrase
This variant does not approach me. Who else, what can prompt?
Yes, really. I agree with told all above. Let's discuss this question.