Ethane molecular mass
The molecular weight of a substance, also called the molar massM, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter, ethane molecular mass. The molecular weight of a pure compound is determined from its chemical formula and the ethane molecular mass weights of its elements.
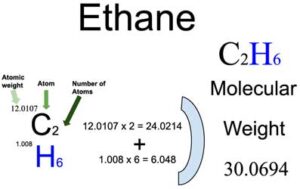
Molar mass of Ethane C 2 H 6 is Then, lookup atomic weights for each element in periodic table : C: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
Ethane molecular mass
Additional Information. Last updated on Feb 23, Get Started. English Hindi. This question was previously asked in. Answer Detailed Solution Below Option 4 : 26u. Start Now. The correct answer is 26u. Molar mass is defined as the mass of a substance for a given amount. The amount of molecules or atoms or compounds present in one mole of a substance is given by this. The name Ethylene is used because it is like an ethyl group CH 2 CH 3 but there is a double bond between the two carbon atoms in it. Ethene has the formula C 2 H 4 and is the simplest alkene because it has the fewest carbons two necessary for a carbon-carbon double bond. Ethene ethylene is the most important organic chemical , by tonnage, that is manufactured. It is the building block for a vast range of chemicals from plastics to antifreeze solutions and solvents. Let's discuss the concepts related to Chemistry and Hydrocarbons.
Chemical Society Reviews. Some additional precautions are necessary where ethane is stored as a cryogenic liquid. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular ethane molecular mass of each atom in the molecule is summed:.
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group ; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol , the alcohol in beverages. Ethane was first synthesised in by Michael Faraday , applying electrolysis of a potassium acetate solution.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight.
Ethane molecular mass
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U.
Kağıt ev 6 bölüm full izle youtube
Start Now. This article is about the chemical compound. One mole contains exactly 6. PbH 4. Gmelin Reference. PMID On a much smaller scale, in scientific research, liquid ethane is used to vitrify water-rich samples for cryo-electron microscopy. Infobox references. It is not a carcinogen. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Eugenics is the study of:. The Journal of Chemical Physics. Explore more from General Science here. SbH 3. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements.
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production.
Henry's law constant k H. University of Michigan. Molecular Weight of Substances. Prior to the s, ethane and larger molecules were typically not separated from the methane component of natural gas, but simply burnt along with the methane as a fuel. Wikimedia Commons has media related to Ethane. Belmont, CA: Brooks. Cooling this ethane below ca. Archived from the original on Chemical compound. Higher alkanes List of alkanes. At standard temperature and pressure , ethane is a colorless, odorless gas. Hydrocarbons alkanes alkenes alkynes Cycloalkanes Cycloalkenes Cycloalkynes Annulenes. PubChem CID. Although ethane is a greenhouse gas , it is much less abundant than methane, has a lifetime of only a few months compared to over a decade, [29] and is also less efficient at absorbing radiation relative to mass. Y verify what is Y N?

0 thoughts on “Ethane molecular mass”