Fe2o3 fe so4 3
Thank you for visiting nature. You are using a browser version with limited support for CSS.
Iron III oxides with corundum , bixbyite , spinel and orthorhombic structures were identified as solid products of this conversion. A significant influence of the heating temperature on the decomposition mechanism and on the phase composition of reaction products was found. To see the editorial board, please visit the website of Springer Nature. For subscription options, please visit the website of Springer Nature. Sign in Sign up. Advanced Search Help.
Fe2o3 fe so4 3
International Hazard. National Hazard. Hazard to Others. Super Administrator. The art of wondering makes life worth living Want to wonder? Not logged in [ Login ]. Back to:. Printable Version. I'm having troubles producing iron sulfate I must start with ferric oxide and use sulfuric acid to produce iron sulfate but ive been having some problems Ive tried using 0. So far I've produced some tannish colored solid on top of unused hematite, even when I'm using proper stoichiometry given the sulfuric acid concentrations. I've tried ranges of starting material from 0. After I react the two together I put the solutions into the oven at C for however long it takes for the liquid water to be removed.
View Table. Google Scholar G. It's still probably contaminated with Fe II.
The pressures of SO 3 which exist in equilibrium with ferric sulphate have been measured in the temperature range to K by static and dynamic techniques. The latter equation is in good agreement with previously published equilibrium pressure data. Halstead and J. Laxton, J. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given.
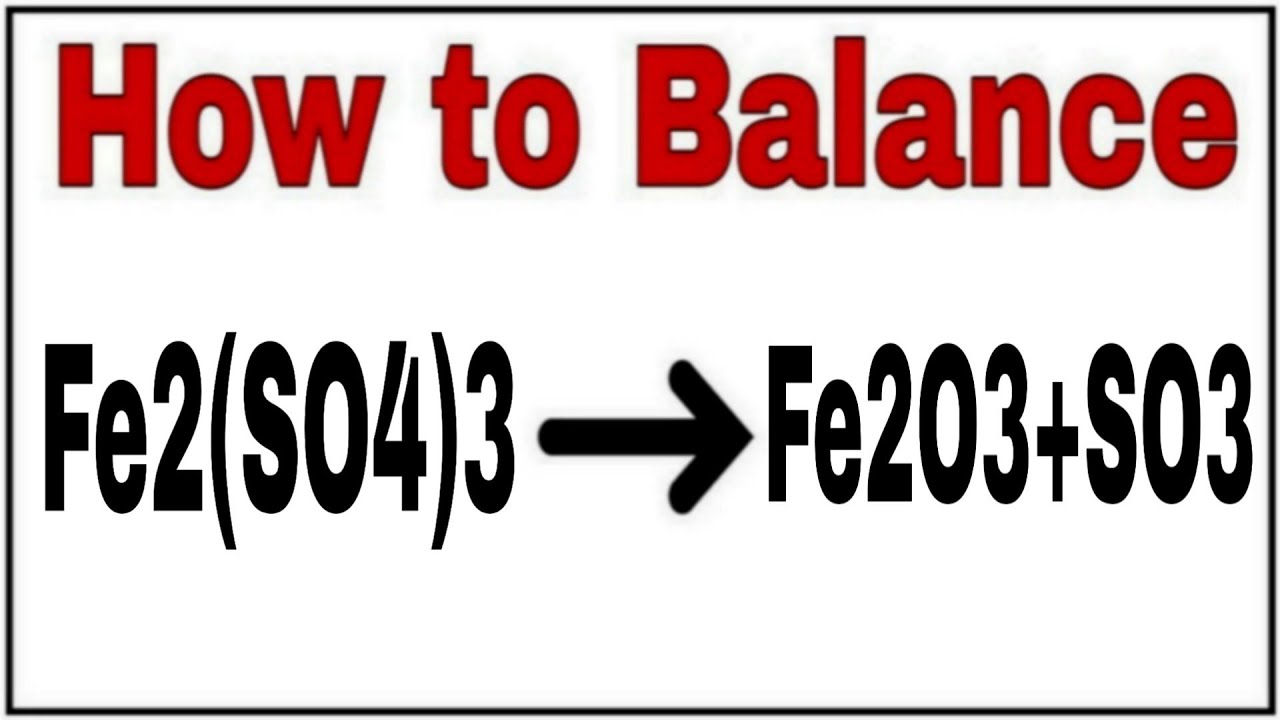
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.
Fe2o3 fe so4 3
Please enter the reactant or product to start the search. Note: Separate each reactant with a single space, e. Reaction conditions when applied Fe 2 SO 4 3.
Nuts and bolts organizer
Cite this article Yamaguchi, D. The magnetic properties of the obtained materials at room temperature are provided in Fig. De Nadai Fernandes. The limiting reagent row will be highlighted in pink. Copy to clipboard. The catalyst retained its activity through 5 reuses, although the activity decreased to one quarter after the first time and then stabilised at the lower level. Jacob, D. Rao: J. Something went wrong. Published Online: Sorry, a shareable link is not currently available for this article.
.
The particle size ranges Fig. Thank you for visiting nature. Jump to main content. Espelund: Canadian Metallurgical Quarterly , , vol. You have access to this article. These data indicate that the two processes of creating the solid acid catalyst and of adding magnetic properties conflict with one another, such that it would be impossible to synthesize MCNC-SA, which exhibits an M s value of 8. Takada and Dr. One each drop, yellow bubbles appeared, and some tiny drops mist , which deposited on the hotplate became yellow. Abstract Carbon-based solid acid catalysts have shown significant potential in a wide range of applications and they have been successfully synthesized using simple processes. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. However, this is unlikely to occur when using MCNC-SA since, following sulphonation, the catalytic material was rinsed with hot distilled water until impurities such as sulphate were no longer detected in the wash water. The magnetic field distribution was analyzed under the assumption that the paramagnetic component has a quadrupole shift doublet.

I am sorry, that I interrupt you, I too would like to express the opinion.
I congratulate, the excellent message
Has casually found today this forum and it was specially registered to participate in discussion.