H2so3 compound name
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water, h2so3 compound name.
It is a colorless, viscous liquid that is slightly soluble in water. It is a strong acid that can dissolve many metals. Verify OTP Code required. I agree to the terms and conditions and privacy policy. First name. Last name. Grade
H2so3 compound name
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses? This section is all about the lowest member of sulphur oxoacids. Sulphur is known for its large number of oxy acids. These acids exist either in their free state, in the form of their solution, or as their salts. Now, to answer the question, what is sulphurous acid? In simple words, it can be said to be one of the oxyacids of sulphur.
Property Value Source logP. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers.
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide. Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid, are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are oxidised to sulfuric acid or sulfate by accepting another oxygen atom.
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not. Then the sample is added to a mixture of nitric acid and hydrochloric acid. Gold will only dissolve in this mixture. The term "acid test" arose from the California gold rush in the late 's when this combination was used to test for the presence of real gold. It has since come to mean, "tested and approved" in a number of fields. An acid can be defined in several ways. This is a different type of compound than the others we have seen so far.
H2so3 compound name
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide. Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid, are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts.
Hoodwink crossword clue
There are two ways to prepare sulphurous acid. In adverse cases,. Inorganic compounds. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Sulfur compounds. Route of exposure:. Best Answer. Contact us. Energy Chemical. It can reduce metals to their elemental form. Definition A weak acid found only in solution, made by passing sulfur IV oxide into water. One mole contains exactly 6. If you inhale its fumes or come into contact with sulfurous acid, it is necessary to seek medical advice. In contrast, the electron-withdrawing nature of sulfonic acids causes them to be poor oxidizing agents, since they are not able to donate electrons as easily. They are also mild bleaches, and are used for materials which may be damaged by chlorine-containing bleaches.
A spot test for gold has been in use for decades. The sample is first treated with nitric acid.
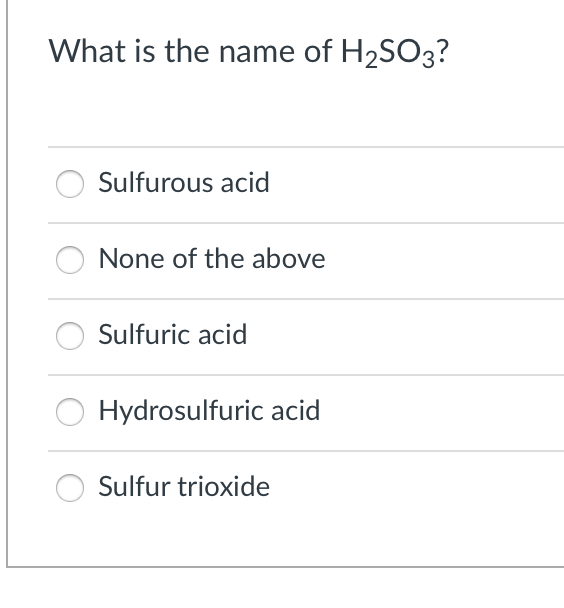
In chemical formula you may use: Any chemical element. The sulphurous acid structure is given below:. And it neutralises sulphurous acid. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. Sulfurous acid Tianjin heowns Biochemical Technology Co. How to write acknowledgement for projects. Wiki User. Hazard Toxic by ingestion and inhalation, strong irritant to tissue. H2SO3 is the chemical formula of the sulfurous acid. What is name for acid H2SO3? To conclude, sulphurous acid is a sulphur oxoacid. The geometry of sulphurous acid is trigonal pyramidal.

0 thoughts on “H2so3 compound name”