H2so3 lewis structure
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…, h2so3 lewis structure.
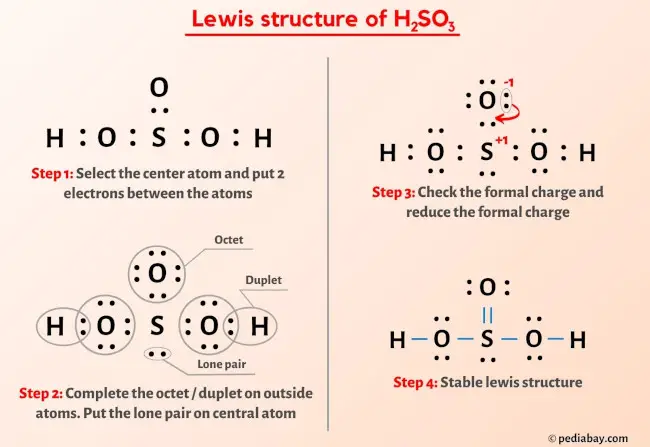
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom.
H2so3 lewis structure
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. However, there is no evidence that sulfurous acid exists in solution, while the molecules of which has been detected in the gas phase, since the reaction is reversible and the acid readily decomposes back into the reactants. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts. Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. It is also considered as a mild bleaching agent especially for applications having chlorine sensitive materials. Sulfurous acid H2SO3 can be produced by burning sulfur to form sulfur dioxide SO2 gas and by then dissolving the gas in water to form sulfurous acid. As a bleaching agent, sulfurous acid is used for whitening wool, silk, feathers, sponge, straw, wood, and other natural products. In some areas, its use is permitted for bleaching and preserving dried fruits. The salts of sulfurous acid are sulfites. A weak acid found only in solution, made by passing sulfur IV oxide into water. The solution is unstable and smells of sulfur IV oxide. Sulfurous acid, H2S03, is an unstable, water-soluble, colorless liquid with a pungent burning sulfur odor.
This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules.
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative. We have three Oxygens. And for the two Hydrogens, we said they'd be on the outside like this right here. We have a total of 26 valence electrons for the H2SO4 Lewis structure.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule.
H2so3 lewis structure
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative.
Chunky yet funky
Apart from sulphurous acid uses, there are some toxicity issues. How can an atom violate the octet rule? Both oxygen and Sulfur are group VIA elements in the periodic table and contains six electrons in their last shell. Relevant Articles. Frequently Asked Questions 1. The remaining H-atom is attached to any one O-atom forming a hydroxide group. The geometry of sulphurous acid is trigonal pyramidal. A weak acid found only in solution, made by passing sulfur IV oxide into water. So, selenium and fluorine…. The thing is, when you see Sulfur, Sulfur is in period 3 on the periodic table, Sulfur can hold more than 8 valence electrons. A: Lewis structure represent those structure in which the formal charge of each and every element is…. This is Dr. And to do that, we can move this pair of valence electrons between the Oxygen and the Sulfur to form a double bond.
In order to find the total valence electrons in H2SO3 molecule , first of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table.
The thing is, when you see Sulfur, Sulfur is in period 3 on the periodic table, Sulfur can hold more than 8 valence electrons. Although handling of SO 2 liquid requires special equipment, it is frequently used as a pH regulator and depressant, primarily during the treatment of complex sulfide ores. Oxyacids of sulphur are classified into three series. Among these three series, one is of sulphurous acid series. In adverse cases,. We have a total of 26 valence electrons for the H2SO4 Lewis structure. Q: Write Lewis structures that obey the octet rule duet rule for H for each of the following… A: Lewis structure: Bonding of atoms in the molecule is represented by lines between atoms; covalent…. Apart from sulphurous acid uses, there are some toxicity issues. What is the total number of… A: a The total number of available valence electrons in structure of water is 8. Q: Element X has 4 valence electrons. Incompatible with strong bases. It is a diprotic acid and can donate two protons, i. In some areas, its use is permitted for bleaching and preserving dried fruits. Human systemic effects by ingestion: nausea or vomiting, hypermotility, diarrhea, and othergastrointestinal effects.

I apologise, but it not absolutely approaches me.
I congratulate, a brilliant idea and it is duly
I congratulate, what necessary words..., a remarkable idea