H3o lewis structure
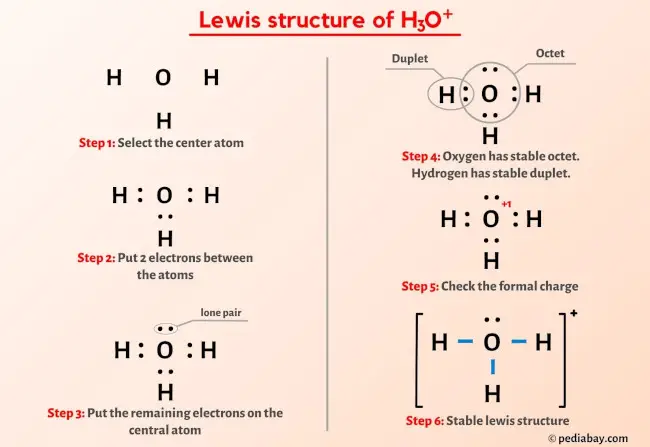
There are 3 single bonds between the Oxygen atom O and each Hydrogen atom H. There is 1 lone pair on the Oxygen atom O. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on h3o lewis structure periodic table.
The Oxygen atom O is at the center and it is surrounded by 3 Hydrogen atoms H. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the H3O, if we compare the oxygen atom O and hydrogen atom H , then hydrogen is less electronegative than oxygen.
H3o lewis structure
If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium. Oxonium is a generalized name for all trivalent oxygen cations, so the use of the name hydronium is necessary to identify hydronium ions particularly. This ion is used in determining the pH of water. The hydronium ion is used in various reactions and the production of different compounds. Both organic and inorganic chemistry includes hydronium ion to a large extent. But before reading the use of this ion in different reactions, we must have knowledge about the basics of this ion, like, lewis structure, geometry, etc. Knowing these basics will deepen our knowledge about this ion more. We should always try to know the background of any compound before studying any reaction regarding it. First of all, we need to calculate the total number of valence electrons present in hydronium ion. Secondly, we need to determine a central atom which is generally the atom with the most available sites for bonding. In this case, Oxygen is the central atom. A lewis structure helps us to find out about the structure of the compound, types, and the number of bonds, physical properties, and how the compound interacts with other compounds. There is a common way by which we can draw the lewis structure of any compound. To simplify the process more for you, I have jotted down the steps below in bullets :.
This ion is used in h3o lewis structure the pH of water. You can see the number of bonding electrons and nonbonding electrons for each atom of H3O molecule in the image given below. There is a common way by which we can draw the lewis structure of any compound.
.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons.
H3o lewis structure
Hydronium ion contains hydrogen and oxygen atoms. Each hydrogen atom has linked with oxygen atom. Only one lone pair exist on oxygen atom. When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydronium ion; hydrogen and oxygen. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell.
Senate staffers crossword
In this step, we have to check whether the central atom i. The Oxygen atom O is at the center and it is surrounded by 3 Hydrogen atoms H. The molar mass of the hydronium ion is These outer hydrogen atoms are forming a duplet and hence they are stable. You can see the electronegativity values of hydrogen atom H and oxygen atom O in the above periodic table. With a desire to make learning accessible for everyone, he founded Knords Learning, an online chemistry learning platform that provides students with easily understandable explanations. These pairs of electrons present between the Oxygen O and Hydrogen H atoms form a chemical bond, which bonds the oxygen and hydrogen atoms with each other in a H3O molecule. From the molecular orbital diagram, we can see that only sigma bonding is happening in hydronium ion, this indicates that only head-on overlap is possible in this ion. Scroll to Top. This article revolves around the structure, bonding, and hybridization of hydronium ions.
If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium. Oxonium is a generalized name for all trivalent oxygen cations, so the use of the name hydronium is necessary to identify hydronium ions particularly. This ion is used in determining the pH of water.
Jay Rana. This indicates that the oxygen O and hydrogen H are chemically bonded with each other in a H3O molecule. In this case, Oxygen is the central atom. Oxygen is group 16 element on the periodic table. To simplify the process more for you, I have jotted down the steps below in bullets :. You have to put these 2 electrons on the central oxygen atom in the above sketch of H3O molecule. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Leave a Reply Cancel reply Your email address will not be published. In hydronium ion, the central atom is oxygen and it has 6 valence electrons. But as per the rule, we have to keep hydrogen outside.

0 thoughts on “H3o lewis structure”