Hcl plus naoh
Key Points. Additional Information.
Wiki User. The product is sodium chloride. The reactants are NaCl and H2O. NaCl and H2O. It is called an acid-base reaction. The product is called a salt. HCl is the acid.
Hcl plus naoh
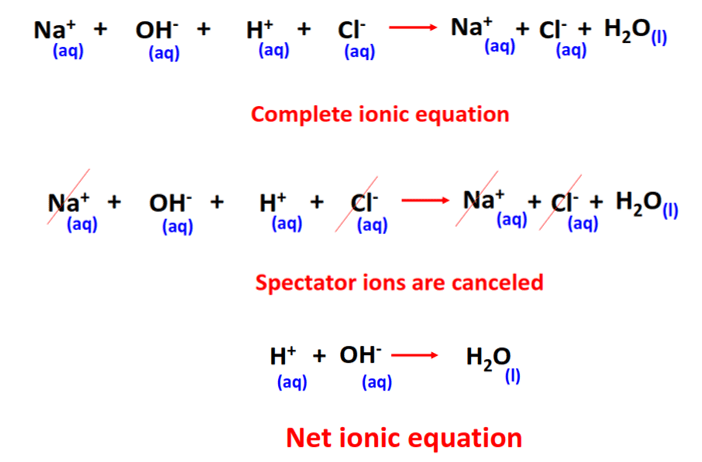
The thing to recognize here is the fact that you're dealing with a neutralization reaction that features sodium hydroxide, "NaOH" , a strong base , and hydrochloric acid, "HCl" , a strong acid. This tells you that the two reactants will dissociate completely in aqueous solution to produce cations and anions. More specifically, you will have. Now, when these two solutions are mixed, the hydroxide anions produced by the strong base and the hydrogen ions produced by the strong acid will neutralize each other to produce water. The sodium cations and the chloride anions act as spectator ions because they are present on both sides of the chemical equation as ions. You can tell that this is the case because sodium chloride, "NaCl" , one of the two products of the reaction, is soluble in aqueous solution. In this case, the dissociation of hydrochloric acid is represented by the chemical equation. How do you write a total ionic equation and a net ionic equation for this reaction? Stefan V. Dec 6, Here's how you can do that. Explanation: The thing to recognize here is the fact that you're dealing with a neutralization reaction that features sodium hydroxide, "NaOH" , a strong base , and hydrochloric acid, "HCl" , a strong acid.
The reactants are NaCl and H2O. SSC Selection Post. JNU Junior Assistant.
Hint: This graph cannot be done by Excel. Select all that apply. NaOH is a strong base and HCl is a strong acid. Thus, the pH of the resultant solution will be determined based on the amount of excess reagent. When doing pH calculations for titrations, the most commonly made mistake is forgetting to take into account the change in volume.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Hcl plus naoh
As we have seen in the section on chemical reactions, when an acid and base are mixed, they undergo a neutralization reaction. This is sometimes true, but the salts that are formed in these reactions may have acidic or basic properties of their own, as we shall now see. A solution is neutral when it contains equal concentrations of hydronium and hydroxide ions. When we mix solutions of an acid and a base, an acid-base neutralization reaction occurs. However, even if we mix stoichiometrically equivalent quantities, we may find that the resulting solution is not neutral. It could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed determines whether the solution is acidic, neutral, or basic. The following four situations illustrate how solutions with various pH values can arise following a neutralization reaction using stoichiometrically equivalent quantities:. Our stomachs contain a solution of roughly 0. The burning sensation associated with heartburn is a result of the acid of the stomach leaking through the muscular valve at the top of the stomach into the lower reaches of the esophagus.
Pokemon platinum exp share
West Bengal Judicial Service. Uttarakhand Assistant Agriculture Officer. Maharashtra Forest Department Stenographer. Bihar Secondary Teacher. Chhattisgarh Junior Engineer. The cooking gas is mainly a mixture of the following two gases:. It is called an acid-base reaction. MP Police Constable. TN Forest Guard. Railway TTE.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
SBI SO. Maharashtra Forest Department Lekhpal. Question bc Heat is evolved during. HAL Design Trainee. Which gas is liberated when zinc acid zincate. Which gas is used for the preparation of soda water? Odisha Police ASI. Airforce Group Y. Speed of sound is highest in which medium? The reason for this colour change is neutralisation of a base NaOH by an added acid.

Between us speaking, in my opinion, it is obvious. I will not begin to speak on this theme.
Completely I share your opinion. In it something is also idea excellent, I support.
It seems excellent idea to me is