Hf molecular geometry
Wiki User, hf molecular geometry. Fluorine is an element, the symbol F would indicate its atomic form not a molecule, the symbol F2 would indicate its diatomic molecular form. Fluorine gas is the F2 diatomic molecular form not F. Lithium is a metal, so is referred to as a metallic lattice, so molecular formula doesnt apply.
Wiki User. Molecular geometry will be bent, electron geometry will be trigonal planar. The only possible geometry of a diatomic molecule such as P2 is linear. Trigonal planar. HF is not an oxyacid; no oxygen in the molecule.
Hf molecular geometry
Buxton , P. Aldrich , J. Shea , A. Legon , W. Flygare; The rotational spectrum and molecular geometry of the cyclopropane—HF dimer. The geometry of the dimer has unambiguously been shown to have HF perpendicular to the cyclopropane edge and in the plane of the ring. HF, on average, lies on the C 2 symmetry axis of the dimer which is additionally the a principal axis. Hyperfine structure due to the spin—spin interaction in HF and DF was resolved as well as the hyperfine structure due to the nuclear quadrupole moment of deuterium in the cyclopropane—DF species. Sign In or Create an Account. Search Dropdown Menu. Advanced Search Citation Search. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 75, Issue 6. Article Navigation.
The only possible geometry of a diatomic molecule such as P2 is linear. This Site.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U.
Hf molecular geometry
Hydrogen fluoride is a colorless liquid or a gaseous compound having the chemical formula HF. It tends to dissolve in water and the colorless aqueous solution is known as hydrofluoric acid. HF has a wide range of applications. It acts as a precursor to the halogen fluorine via electrolysis procedure. Other than this, it also acts as the precursor of several metal fluorides like aluminum fluoride and uranium hexafluoride. Anhydrous HF has catalyzing properties and is hence used in the process of petroleum alkylation to increase the octane number of petroleum. Also, hydrogen fluoride can be used to manufacture herbicides, fluorescent light bulbs, refrigerants, and so on. Chemical bonding is the study of atomic attraction that results in the formation of new products. The science behind the formation of chemical bonds helps us understand several chemical and physical properties exhibited by different molecules and compounds.
White gift boxes spotlight
Flygare, J. Search Dropdown Menu. Flygare; The rotational spectrum and molecular geometry of the cyclopropane—HF dimer. Research Article September 15 What is the structural geometry of the NOF molecule? What general patterns for identifying the electron geometry of a molecule? Google Scholar. Still have questions? All Rights Reserved. Sign in Don't already have an account? Find more answers Ask your question. Arrange F2 F2- and F2 plus in order of decreasing stability?
.
Fluorine gas is the F2 diatomic molecular form not F. Search Dropdown Menu. HF is not an oxyacid; no oxygen in the molecule. F2 has a linear shape. Search ADS. The F2 key. Which compound has the highest melting point? Shea ; J. Wiki User. More answers. CrossRef Aldrich , J. Best Answer.

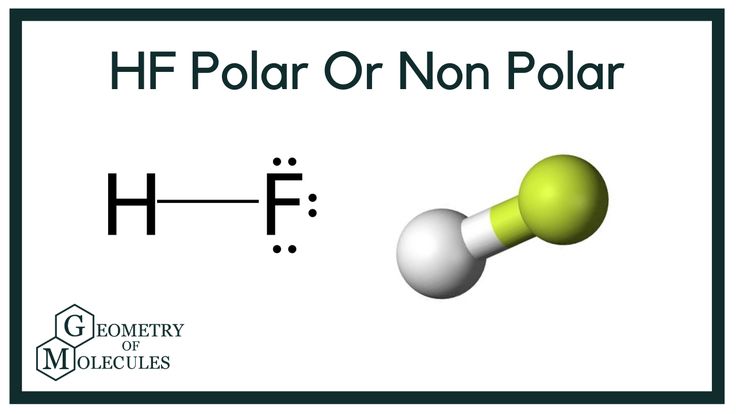
0 thoughts on “Hf molecular geometry”