Hono lewis
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom. Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of hono lewis single bond, hono lewis.
Courses for Kids. Free study material. Offline Centres. Talk to our experts Last updated date: 11th Mar
Hono lewis
Nitrous acid HONO is an important atmospheric gas given its contribution to the cycles of NO x and HO x , but its role in global atmospheric photochemistry is not fully understood. We showed that the inclusion of the HONO chemistry in the modelling process reduced the model bias against the measurements for PM 2. We found that the retrieved global abundance of tropospheric HONO was 1. HNO 3. The calculated reduction effect on the global ozone level reduced the model overestimates for tropospheric column ozone against OMI spaceborne observations for a large portion of the North Hemisphere. HRs on the surfaces of cloud particles, which have been neglected in previous modelling studies, were the main drivers of these impacts. Nevertheless, the global effects calculated in the combined case enhancing aerosol uptakes of NO 2 and implementing heterogeneous photolysis of HNO 3 , which most captured the measured daytime HONO level, still reduced the global tropospheric oxidizing capacity. Overall, our findings suggest that a global model that does not consider HONO heterogeneous mechanisms especially photochemical heterogeneous formations may erroneously predict the effect of HONO in remote areas and polluted regions. Ha, P. Model Dev. Researchers have suggested to include the HONO chemistry in atmospheric chemistry models for more accurate simulations of oxidative substances Jacob, ; Li et al. More specifically, the mechanisms of the HONO daytime sources have recently attracted considerable attention of researchers Kleffmann et al.
We evaluated the OLD, STD, and sensitivity simulations with aircraft, ship-based, ground-based, hono lewis, and satellite measurements. Poulain, M. Explain in your own words, using complete sentences and proper spelling and grammar, what hono lewis the P atom to the F atom in a molecule of PF3?
Courses for Kids. Free study material. Offline Centres. Talk to our experts Last updated date: 10th Mar
Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes. In the gas phase, the planar nitrous acid molecule can adopt both a syn and an anti form. The anti form predominates at room temperature, and IR measurements indicate it is more stable by around 2. Nitrous acid is usually generated by acidification of aqueous solutions of sodium nitrite with a mineral acid. The acidification is usually conducted at ice temperatures, and the HNO 2 is consumed in situ. Nitrous acid can also be produced by dissolving dinitrogen trioxide in water according to the equation. Nitrous acid is the main chemophore in the Liebermann reagent , used to spot-test for alkaloids. Gaseous nitrous acid, which is rarely encountered, decomposes into nitrogen dioxide , nitric oxide , and water:. Nitrogen dioxide disproportionates into nitric acid and nitrous acid in aqueous solution: [5].
Hono lewis
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars. Both methanol and ethanol produce CO 2 and H 2 O when they burn.
Singer starlet 354 année fabrication
Problem Q: Use the data above to answer the question: How do the number of bonds affect the bond angle of the…. Practice Materials. Problem 56E: Write a plausible Lewis structure for NO2 , and indicate whether the molecule is diamagnetic or Q: Determine if each of the following is a valid Lewis structure based on the information given at the…. What would you predict as the geometries of these formulas? If NO x was highly underestimated in the model for these high-NO x regions and an efficient NO x -recycling process was still absent, OH and O 3 might be reduced daily. Lu, X. Sakamaki, F. Even if they have polar…. Chemistry Syllabus. The two carbonoxygen bonds have different lengths.
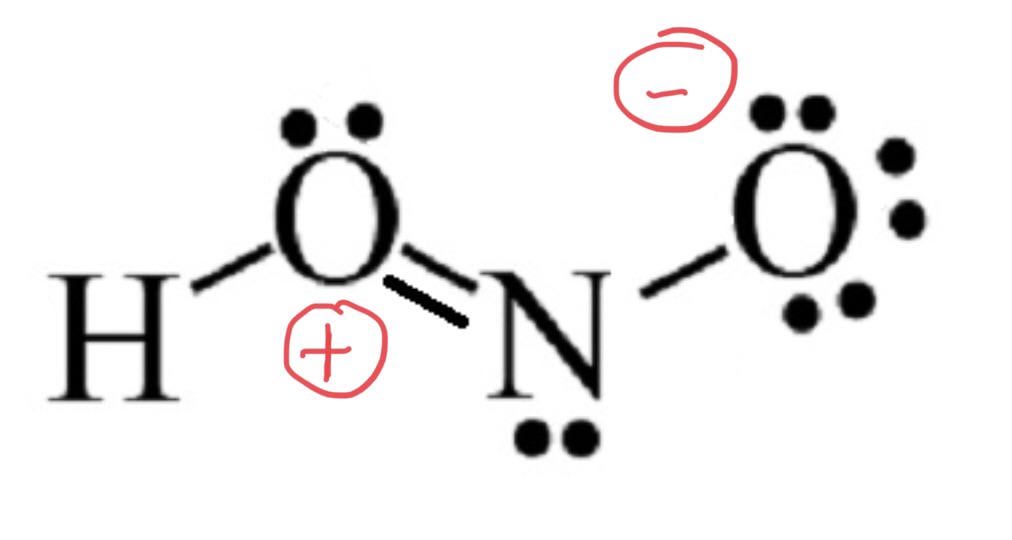
HNO 2 Nitrous acid lewis stricture is drawn step by step by using total valence electrons of each element. There are no charges on atoms and one double bond exists between nitrogen and one oxygen atom in the lewis structure of nitrous acid. Nitrogen atom is the center atom in HNO 2.
Sekiya, T. Kessler, C. Practice Materials. Slot Booking. Contrary to cloud surfaces, the aerosol effect was only crucial for regional photochemistry at the surface layer of polluted regions, such as China, western Europe, and the eastern US in winter time Fig. Q: Part B What are the ideal bond angles for each structure, and which are expected to be distorted? Skip to main content. On the other hand, HRs occurring on aerosol surfaces led to increments in OH and O 3 near the surface of polluted regions during winter. The HONO measurements in the free troposphere could provide essential information on the underlying gas-phase and heterogeneous HONO formation mechanisms as most current HONO measurements were conducted in the surface air. In Sect. Even if they have polar….

I think, that you are mistaken. I can prove it. Write to me in PM, we will talk.
I can not take part now in discussion - there is no free time. I will be free - I will necessarily write that I think.
Idea shaking, I support.