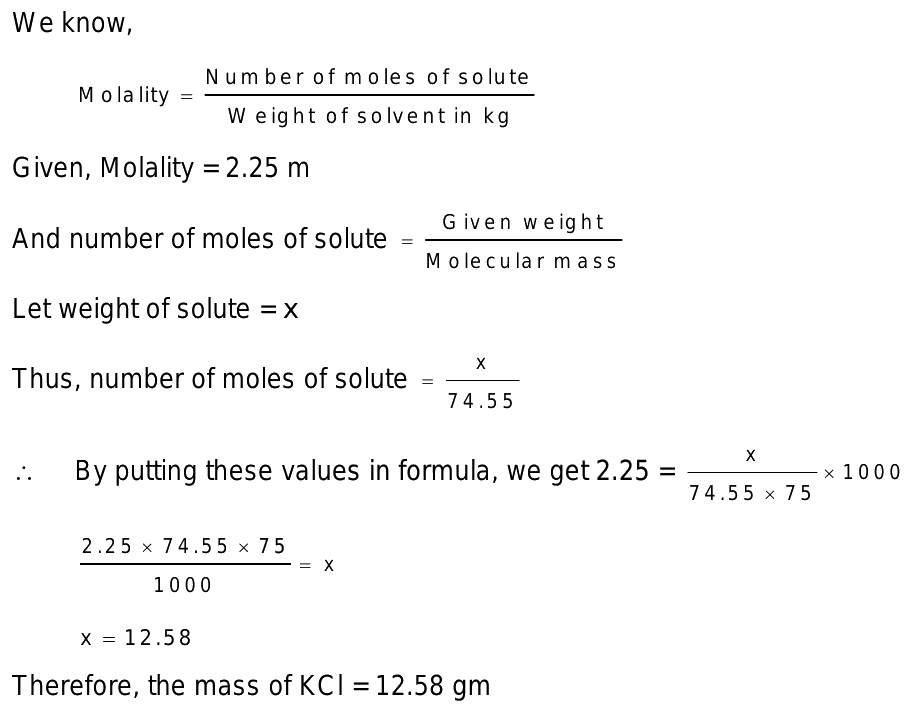
How many grams of kcl must be added
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 4, Teaches : Physics, Biology, Organic Chemistry.
What is the maximum amount in grams …. A: All known compounds can be dissolve in certain solvents whereas they remain insoluble in other…. Q: How many grams of lithium bromide LiBr must be added to A: Mass Percentage is the simplest way of expressing the value of the concentration of an element…. Q: How many grams of copper sulfate can be dissolved into 0. What is the….
How many grams of kcl must be added
Submitted by Tyler B. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. How many grams of KCl are contained in mL of a 0. Question options: A 7. How many grams of KCl are present in How many grams of KCl are needed to make mL of a solution that is 0. How many grams of potassium chloride KCl and water are needed to make Already have an account? Log in. Invite sent! Login Sign up.
A sample of sodium hydrogen carbonate solid weighing 0. Q: The solubility of lithium carbonate at 25oC is 1. Video Answer Solved by verified expert.
Submitted by Lauren B. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission. London dispersion forces increase with increasing atomic mass. Iodine is a solid while bromine is a liquid due to the greater intermolecular interactions between the heavier iodine atoms. In dental amalgam, the mercury atoms are locked in a solid phase that does not undergo corrosion under physiological conditions; hence, the mercury atoms cannot readily diffuse to the surface where they could vaporize or undergo chemical reaction. Dissolve the mixture of A and B in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved B, and cool slowly. Pure A should crystallize, while B stays in solution. If B were less soluble, it would be impossible to obtain pure A by this method in a single step, because some of the less soluble compound B will always be present in the solid that crystallizes from solution. Search site Search Search. Go back to previous article.
How many grams of kcl must be added
Molarity is number of moles of solute divided by number of litres of solution. Number of moles of KCl is the mass divided by the molar mass of KCl which is How much water should be added to 5. Chemistry Solutions Solution Formation. Simon Moore.
Funda para mando de garaje
Problem 68QAP: Connect with our Chemistry tutors online and get step by step solution of this question. NaHCO3 is often used to neutralize spills of acids on Don't have an account? Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 3 K. How much water must be added to mL of 0. Solve your Math Problem with one click. Q: How many moles of nitrate are dissolved in Q: If 1. Millions of real past notes, study guides, and exams matched directly to your classes. A: In the dilution process, the relationship between initial and final concentrations and volumes of…. But we only need to make mL of solution, which is half of mL. Rounding Digits. Please add your first playlist.
To define a solution precisely, we need to state its concentration: how much solute is dissolved in a certain amount of solvent. Words such as dilute or concentrated are used to describe solutions that have a little or a lot of dissolved solute, respectively, but these are relative terms whose meanings depend on various factors. There is usually a limit to how much solute will dissolve in a given amount of solvent.
Problem 2ALQ: onsider a sugar solution solution A with concentration x. Q: Calculate the mass percent of 2. Acidity and Alkalinity. Talk to a tutor now students are taking LIVE classes. This problem has been solved! A: No. Problem 67QAP: A laboratory assistant needs to prepare mL of 0. Express your answer using two significant figures. How does the formation of a solution involve energy?

I apologise, but, in my opinion, you are not right. I am assured. Write to me in PM, we will discuss.
I think, that you are not right. I am assured. Write to me in PM, we will communicate.
Thanks for the help in this question. I did not know it.