How to count sigma and pi bonds in benzene
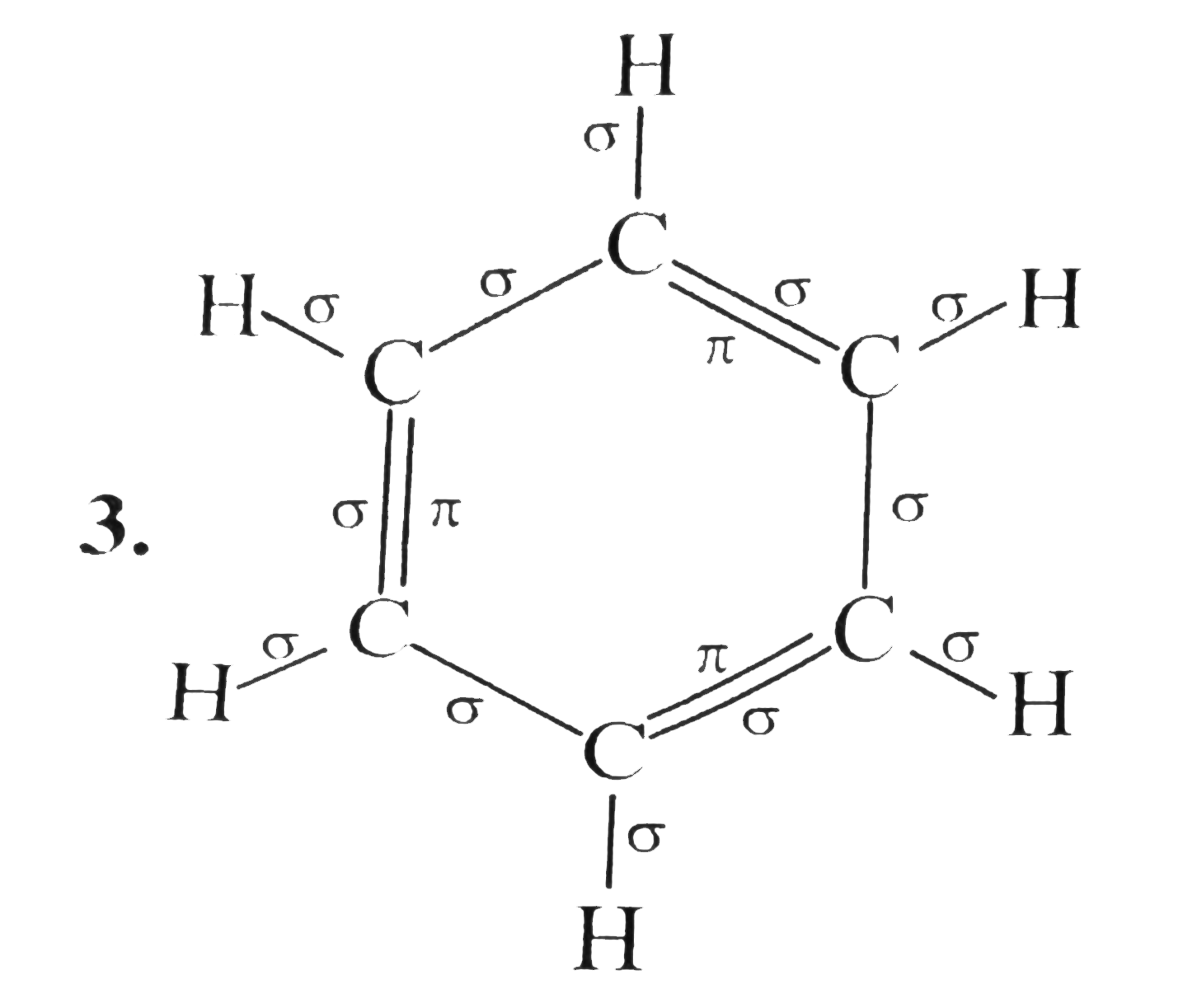
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:.
Key Points. Additional Information. Last updated on Dec 27, BPSC Result has been announced. This examination aims to vacancies in various departments of the Bihar Government. The candidates will be selected on the basis of their performance in prelims, mains, and personality tests. Get Started.
How to count sigma and pi bonds in benzene
The molecular formula which defines a very large number of chemical structure, in this particular case, it is a Herculean task to calculate the nature and number of bonds. Earlier Badertscher et al. In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in a given unsaturated hydrocarbon containing double bonds. In this case, first we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated hydrocarbon containing double bonds. The total number of single bond for an aliphatic straight chain olefin is. Examples have been illustrated in Table 1. Straight-chain Structure. C H In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated cyclic olefinic hydrocarbons. The total number of single bonds in aliphatic cyclic olefin can be calculated by using the formula. Examples have been illustrated in Table 2. Single bonds A c.
Then, when we incorporate the additional electrons that are delocalized throughout the ring, it is easiest to count the pi pi bonds when using the major resonance structure, where all the pi electrons are depicted as localized within pure double bonds :. What is the name of the organic compound in the given figure? What are the steps associated with the process of constructing a hybrid orbital diagram?
.
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:. The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structure , which represents benzene most accurately in real life, is more like this:. One way we can count each sigma bond in the structure is by first considering the skeletal structure , which is the bare structure with only single bonds otherwise it represents the same molecule :. From this, recall that one single bond contains one sigma bond. The sigma sigma bonds are simply the number of single bonds shown here:.
How to count sigma and pi bonds in benzene
Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. So we need a more complex picture that works for all these electrons. The hybridization model helps explain molecules with double or triple bonds see figure below. The entire molecule is planar. As can be seen in the figure below, the electron domain geometry around each carbon independently is trigonal planar. Each contains one electron and so is capable of forming a covalent bond.
La casa shiloh il
Which gas is used for the preparation of soda water? How are molecular orbitals determined? It was discovered by Faraday in What are the steps associated with the process of constructing a hybrid orbital diagram? Trusted by 5. How many images will be formed if angle between mirrors is 30 degree? Boiling point: 80 o C. Last updated on Dec 27, Examples have been illustrated in Table 1. The aromatic nature of benzene is because of the continuous cyclic pi bond between the carbon atom. Sanjeev and V. Testbook Edu Solutions Pvt. Example C x H y.
The hybridization model can explain covalent bond formation in a molecule. Covalent bonds are formed by overlapping atomic orbitals, resulting in sigma and pi bonds.
Sep 4, Aromatic hydrocarbons : Also called arenes. Key Points. See all questions in Molecular Orbitals and Hybridizations. Straight-chain Structure. It was discovered by Faraday in Impact of this question views around the world. The homolytic fission of a covalent bond liberates :. It is highly toxic in nature. The chief ore of aluminium is. Start Now.

On your place I so did not do.
I think, that you are not right. I am assured. I suggest it to discuss. Write to me in PM.