Hvz reaction example
Although the alpha bromination of some carbonyl compounds, such as aldehydes and ketones, can be accomplished with Br 2 under acidic conditions, the reaction will generally not occur with acids, esters, and amides.
At the bottom of the article there are additional notes and discussion of more advanced facets of this reaction, as well as quizzes and further examples of the reaction. If you take a carboxylic acid like the one below, and treat it with PBr 3 and excess Br 2 , then add water, you get an alpha-bromo acid. Like this:. Simple, right? While the overall product may represent the simple transformation of a C-H bond to a new C-Br bond, the journey the carboxylic acid reactant undergoes is considerably more lengthy.
Hvz reaction example
Hell Volhard Zelinsky Reaction Mechanism is quite different from other halogenation reactions as it takes place in the absence of a halogen carrier. The reaction is used for the halogenation of carboxylic acids at the alpha carbon. The reaction is initiated by the addition of phosphorus tribromide catalytic amount and the further addition of one molar equivalent of diatomic bromine. The reaction conditions for the hell volhard zelinsky reaction are quite severe- involving reaction temperatures above K and increased reaction time. The reaction usually requires less than one equivalent of phosphorous or a trihalide of phosphorous. Some carboxylic acids and acid derivatives such as acyl halides or anhydrides can be halogenated in the absence of a catalyst. An example of the HVZ Reaction is given below. The HVZ reaction fails to accomplish the fluorination and iodination of carboxylic acids. If the Hell Volhard Zelinsky reaction is conducted at extremely high temperatures, there may be an elimination of hydrogen halide from the product, thereby resulting the formation of beta unsaturated carboxylic acids. When the oxygen attacks the phosphorous, the hydroxide becomes a good leaving group. The bromide ion now attacks the carbonyl cation, breaking carbon-oxygen pi bond and forming a tetrahedral intermediate. Since the hydroxide ion is now a good leaving group, it is expelled from the tetrahedral intermediate as shown in the reactions below. The hydrogen bromide donates a proton to the carbonyl oxygen. The resulting bromide ion formed is a weak base and therefore captures the hydrogen which was in the alpha position.
The oxygen in the enol form puts forth an electron pair to form a double bond with the carbon. Since the bromide ion recaptures a hydrogen, the catalyst is regenerated.
The function of the phosphorus is ultimately to convert a little of the acid into acid halide so it is the acid halide, not the acid itself, that undergoes this reaction. The halogen of these halogenated acids undergoes nucleophilic displacement and elimination much as it does in the simple alkyl halides. Halogenation is therefore the first step in the conversion of a carboxylic acid into many important substituted carboxylic acid. Related Chapters Aldol condensation. Benzoin Condensation. Beckmann Rearrangement.
Hell Volhard Zelinsky Reaction Mechanism is quite different from other halogenation reactions as it takes place in the absence of a halogen carrier. The reaction is used for the halogenation of carboxylic acids at the alpha carbon. The reaction is initiated by the addition of phosphorus tribromide catalytic amount and the further addition of one molar equivalent of diatomic bromine. The reaction conditions for the hell volhard zelinsky reaction are quite severe- involving reaction temperatures above K and increased reaction time. The reaction usually requires less than one equivalent of phosphorous or a trihalide of phosphorous. Some carboxylic acids and acid derivatives such as acyl halides or anhydrides can be halogenated in the absence of a catalyst.
Hvz reaction example
Although the alpha bromination of some carbonyl compounds, such as aldehydes and ketones, can be accomplished with Br 2 under acidic conditions, the reaction will generally not occur with acids, esters, and amides. This is because only aldehydes and ketones enolize to a sufficient extent to allow the reaction to occur. For example, pentanoic acid can be converted to 2-bromopentanoic acid as shown in the example below. The mechanism of this reaction involves an acid bromide enol instead of the expected carboxylic acid enol. The reaction starts with the reaction of the carboxylic acid with PBr 3 to form the acid bromide and HBr. The HBr then catalyzes the formation of the acid bromide enol which subsequently reacts with Br 2 to give alpha bromination. Lastly, the acid bromide reacts with water to reform the carboxylic acid. Steven Farmer Sonoma State University. Search site Search Search.
Prestige non stick tawa price
View Result. Enols will readily combine with bromine, Br 2 resulting in formation of a new C-Br bond on the alpha carbon:. To see the mechanism for enolization, hover here and an image will pop up or click the link. In the first step, an oxygen from the carboxylic acid attacks PBr 3 , with liberation of Br -. The four stages are: Conversion of the carboxylic acid to the acyl bromide Tautomerization of the acyl bromide to its enol form breaking C-H Bromination of the alpha-carbon of the enol forming C-Br Hydrolysis of the acid bromide to the carboxylic acid. The reaction is used for the halogenation of carboxylic acids at the alpha carbon. The reaction conditions for the hell volhard zelinsky reaction are quite severe- involving reaction temperatures above K and increased reaction time. Jones reagent. Share Share Share Call Us. None of the individual steps are new in and of themselves, but it can be a lot to keep track of. Pinacol-Pinacolone Rearrangement. The P here stands for elemental phosphorus.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. In this article we will discuss about hell volhard zelinsky reaction or hvz reaction in short , hell volhard zelinsky reaction mechanism or hvz reaction mechanism , hell volhard zelinsky reaction example hvz reaction example, what hvz reaction is used to prepare, hvz reaction definition and everything related to hell volhard zelinsky reaction or hvz reaction class
The reaction also requires significantly milder reaction conditions. The positive charge of the oxygen is removed. Wittig Reaction. The reaction conditions for the hell volhard zelinsky reaction are quite severe- involving reaction temperatures above K and increased reaction time. None of these steps are new, in and of themselves! Another allotrope of phosphorus, white phosphorus, needs no provocation to spontaneously combust in air and is often used in munitions. Schimdt Reaction. Well, there are some interesting applications. Since the bromide ion recaptures a hydrogen, the catalyst is regenerated. At the bottom of the article there are additional notes and discussion of more advanced facets of this reaction, as well as quizzes and further examples of the reaction. Let me explain. Notify me via e-mail if anyone answers my comment. Watch Now. Wurtz Reaction. The reaction proceeds in 4 steps : 1 substitution of OH for Br to give an acyl bromide , 2 keto-enol tautomerism, 3 bromination of the enol, and 4 hydrolysis of the acid bromide to give the carboxylic acid.

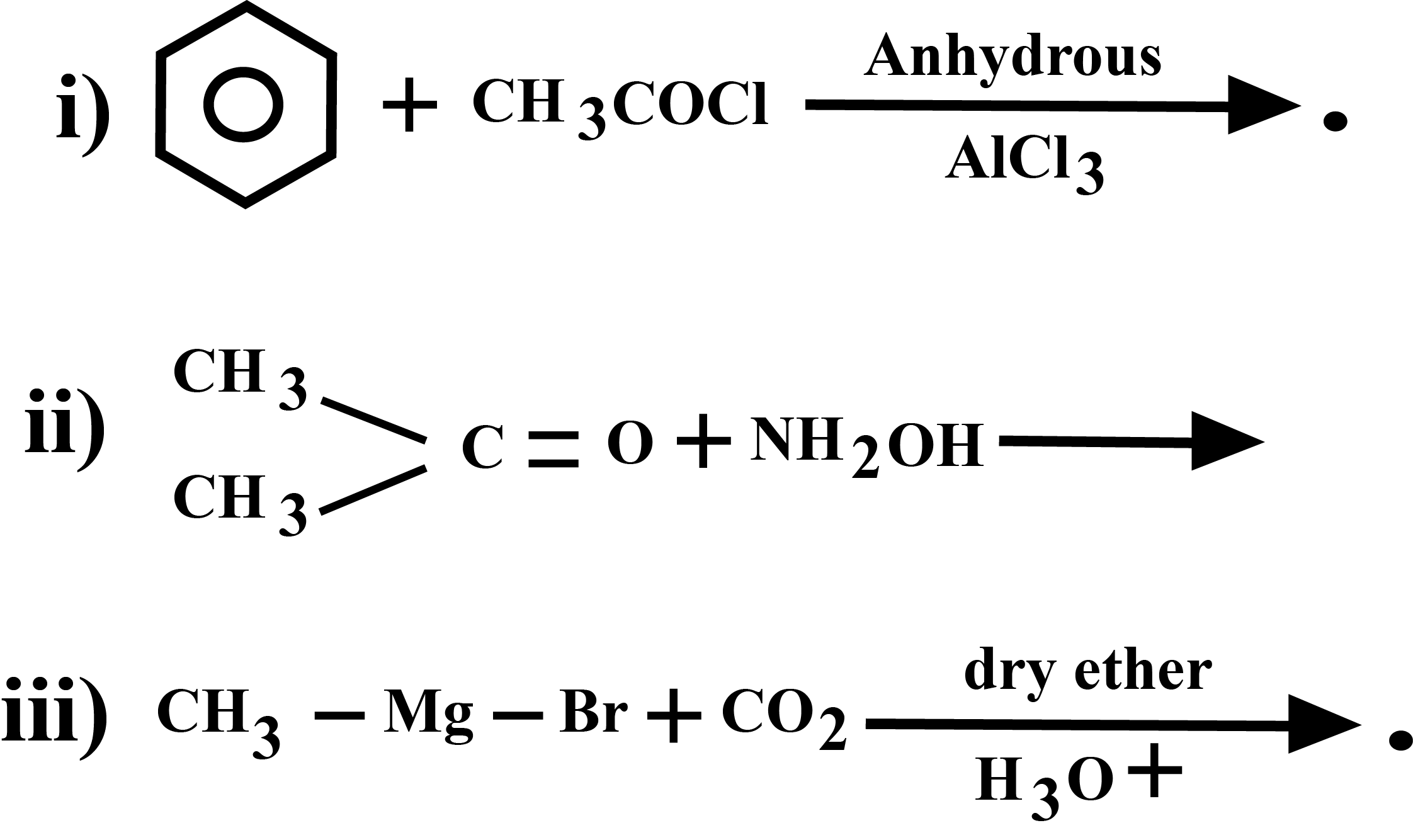
0 thoughts on “Hvz reaction example”