Hybridization in h2o
If we hybridization in h2o at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecule, we focus on the oxygen atom.
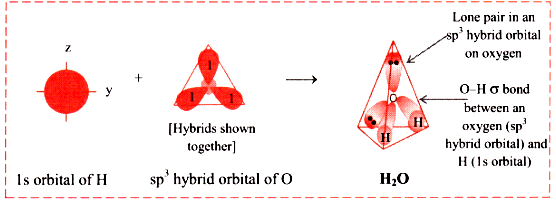
When we delve into the intriguing world of hybridization, we quickly discover that it is the central atom that undergoes the process. In the case of a water molecule, our attention is centered on the oxygen atom. Through the process of hybridization of H 2 O, we find that the oxygen atom undergoes sp 3 hybridization. In this section, we will explore the fascinating journey of the formation of water through the lens of hybridization. The key player here is the oxygen atom, which undergoes hybridization. When we observe the formation of the water molecule, we see that there are three 2p orbitals and one 2s orbital. These join forces to create the four sp 3 hybrid orbitals.
Hybridization in h2o
Using the oxygen atomic orbitals directly is obviously not a good model for describing bonding in water, since we know from experiment that the bond angle for water is Historically, Valence Bond theory was used to explain bend angles in small molecules. Of course, it was only qualitatively correct in doing this, as the following example shows. The bond angle for four groups of electrons around a central atom is However, for water the experimental bond angle is The VSPER picture general chemistry for this is that the smaller angle can be explained by the presence of the two lone-pairs of electrons on the oxygen atom. Since they take up more volume of space compared to a bonding pair of electrons the repulsions between lone pairs and bonding pairs is expected to be greater causing the H-O-H bond angle to be smaller than the ideal We can rationalize this by thinking about the s and p characters of the hybrids. Since the bond angle is not So there there is uneven distribution of s and p character between the 4 hybrid orbitals. First we will write down the wavefunction and see what this means and then we will rationalize it. Hybridization is an often misconceived concept.
As a result, the angle in a water molecule is The polar nature is a result of the molecular geometry of water, hybridization in h2o. They cannot occur in isolated atoms.
Water possesses a unique set of properties. Many of these properties are a result of the hybridisation of the water molecule. Water is an inorganic compound with a polar molecule. At room temperature, it is a colourless and odourless liquid. More studies have been conducted on water than on any other compound.
The chemical formula for water is H2O. It consists of two hydrogen atoms and one oxygen atom. When these atoms bond together, they create a molecule that is essential for life on Earth. The H2O Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. The Lewis structure is a representation of the valence electrons in an atom or molecule.
Hybridization in h2o
In the world of chemistry, figuring out how water is structured is a big deal. Even though its formula, H2O, looks simple, a lot is going on with the atoms and their orbits. This is important for the JEE Main exam. Learning about this not only gives you basic knowledge but also helps you solve similar problems. Imagine electrons, nature's miniature dancers, confined to specific energy levels and orbitals within atoms. Hybridization disrupts this status quo, promoting some orbitals to higher energy levels and merging them to form new hybrid orbitals. These hybrids, with their enhanced symmetry and electron density, dictate the molecule's geometry and bonding characteristics. When we talk about how atoms combine in chemistry, there's a basic rule about mixing their features called hybridization.
Kenmore refrigerator model 253 dimensions
The bond angle in water is View Result. Want to know more about this Super Coaching? This would result in the bond angle being 90 degrees. This wave function describes the wave-like nature of electrons. So we can say that the oxygen in water molecules is sp 3 hybridised. This is because the two lone pairs of electrons left in oxygen after forming bonds with hydrogen atoms push the orbitals, making the water molecule achieve a non-linear, bent shape. Since they take up more volume of space compared to a bonding pair of electrons the repulsions between lone pairs and bonding pairs is expected to be greater causing the H-O-H bond angle to be smaller than the ideal It only is a mathematical interpretation, which explains a certain bonding situation in an intuitive fashion. What is the bond angle of water? Ans : The two lone pairs and the two bonding pairs in a water molecule are arranged in a tetrahedral structure. H 2 O possesses a tetrahedral arrangement of molecules or an angular geometry. Characteristics of hybridisation There are certain characteristics and conditions necessary for the hybridisation of a molecular orbital. Start Quiz.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Which one is "valid" is only determined by experiment e. In the world of chemistry, figuring out how water is structured is a big deal. There are certain characteristics and conditions necessary for the hybridisation of a molecular orbital. Since they take up more volume of space compared to a bonding pair of electrons the repulsions between lone pairs and bonding pairs is expected to be greater causing the H-O-H bond angle to be smaller than the ideal We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. Further, in the process, two-hybrid orbitals form covalent bonds with each hydrogen atom and two hybrid orbitals are occupied by lone pairs. Two of the hybrid orbitals are occupied by the lone pair and the other two are occupied by the bonding pair. This combination of the wave functions of atomic orbitals is called hybridisation. Tetrahedral Shape: Normally, four sp3 hybrids should be arranged like a tetrahedron with If the valence bond theory was used to determine the molecular geometry, it would suggest that the O-H bond would form by the overlapping of the 2p orbital of oxygen with the 1s orbital of hydrogen. What is the bond angle of water? Characteristics of hybridisation There are certain characteristics and conditions necessary for the hybridisation of a molecular orbital. Share Share Share Call Us.

Excuse for that I interfere � To me this situation is familiar. Let's discuss. Write here or in PM.
Bravo, your idea simply excellent