Hydrogen sulfide polar or nonpolar
H 2 S is the chemical formula for the compound hydrogen sulfide.
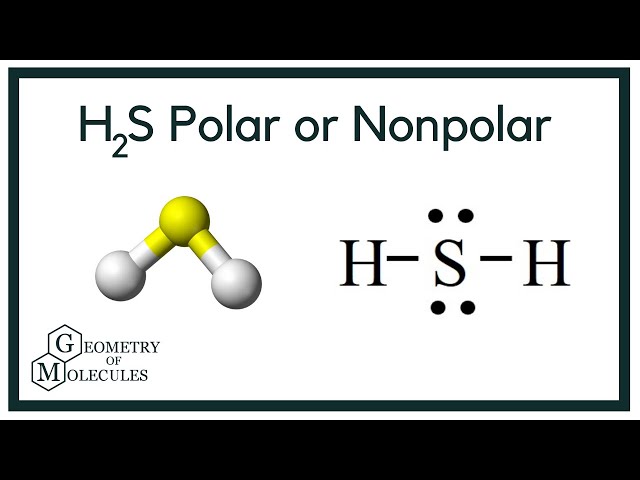
Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. It is poisonous and has a foul odor like a rotten egg. The compound hydrogen sulphide has the chemical formula H 2 S. Hydrogen sulphide is a covalent molecule made up of two hydrogen atoms bound to a sulphur atom in the middle. Hydrogen sulphide, like water H 2 O , is a hydrogen chalcogenide, which is a chemical made up of hydrogen and a group 16 element oxygen, sulphur, selenium, and tellurium. So, is H 2 S polar or nonpolar? H 2 S is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of Hydrogen 2.
Hydrogen sulfide polar or nonpolar
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting. Geometry H2S Polar or Nonpolar.
Hydrogen Sulfide manufacturers. If two elements have an EN difference between 0.
.
Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H 2 S. The molecule has two Hydrogen atoms and a single Sulfur atom. H 2 S is also a precursor for elemental Sulfur. It also plays a vital role in signaling pathways in the human body. So to understand the hybridization, polarity, and molecular geometry of this compound, it is essential to know its Lewis structure. Before knowing its Lewis structure, let us calculate the total number of valence electrons in Hydrogen Sulfide as these electrons participate in bond formation and help us study Lewis structure with ease. To know the total number of valence electrons in Hydrogen Sulfide we need to add the valence electrons of both Hydrogen and Sulfur atoms. There are two atoms of Hydrogen and a single atom of Sulfur in the compound.
Hydrogen sulfide polar or nonpolar
Sulfur compounds are very different in nature and similar to water. Hydrogen sulfide has the chemical formula H2S. All atoms belong to the non-metal family group in the periodic table and possess a high electronegativity value sulfur atom. In this blog post, we are going to discuss the polarity of H2S in a detailed manner. H2S is commonly appearing at ordinary temperatures and pressures, it exists as a gas with a rotten egg smell. H2S contains one sulfur atom and two hydrogen atoms.
Viaport vodafone
Sulfur is slightly more electronegative than hydrogen, so it does pull slightly harder on the shared electrons. The synthesize method of tafenoquine. So, is H 2 S polar or nonpolar? Under the presence of heat and pressure, metal sulfide compounds will undergo hydrolysis with water to form a metal oxide and hydrogen sulfide gas. Post your question. Applying the previous lesson on polarity, we can find out if hydrogen sulfide is a polar compound. The earth;s crust contains large quantities of sulfur and sulfur-containing minerals. When two atoms form a covalent bond, they do so by sharing valence electrons. Geometry H2S Polar or Nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn: Overall, we can say that H 2 S is slightly polar. The EN difference between hydrogen and sulfur is 0.
H2S is a slightly polar molecule because of the small difference in electronegativity values of Hydrogen 2. In addition, the presence of two lone pairs that are on the opposite side of the two Hydrogen atoms also makes the molec ule more polar and causes a bent shape geometrical structure of H 2 S.
Uses of H 2 S It is used to produce hydrogen and sulfuric acid. Mar 8, Is silver chloride soluble in water? Hence, the molecule has an odd distribution of atoms around the central atom making it non-symmetrical. The remaining four electrons go to the sulfur: The central atom has a steric number of 4 — two atoms and two lone pairs. You can also subscribe without commenting. Hydrogen sulfide is combustible and will react with oxygen and heat to form sulfur dioxide and water. So, is H 2 S polar or nonpolar? Conversely, treating metal sulfides with a strong acid results in the production of hydrogen sulfide. It reacts with metal ions to form metal sulfides, most commonly with lead Pb to form lead II sulfide PbS. If the difference is less than 0. If the difference in electronegativity is less than 0. Although hydrogen sulfide is extremely toxic to humans in large quantities, small amounts of hydrogen sulfide play a crucial role in human biology. If the difference in electronegativity is between 0. Thus, hydrogen sulfide is one of the main constituents of the sulfur cycle. Hydrogen Sulfide is a colorless, very poisonous, flammable gas with the characteristic foul odor of rotten eggs.

The amusing information