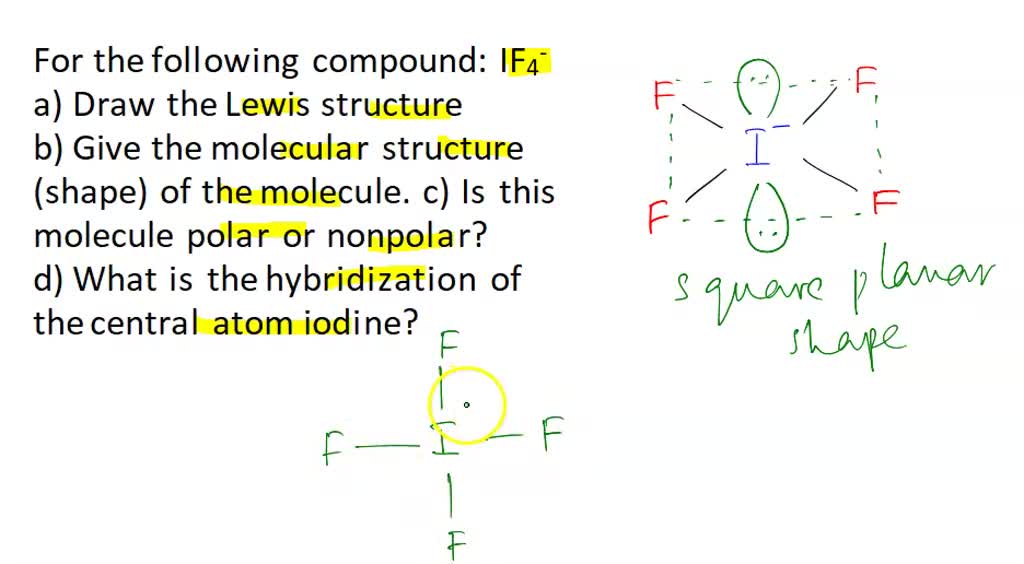
If4 shape
The iodine atom will be the central atom.
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties.
If4 shape
.
More specifically, the repulsion coming from the lone pairs cancels out, if4 shape, since one is pressing down and the other one is pressing up on the bonding electrons.
.
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties.
If4 shape
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
The croods full movie full movie
Writing Ionic Compounds. Power and Root Functions -. Born Haber Cycle. Solution Stoichiometry. Gas Stoichiometry. The central atom is surrounded by 6 regions of electron densiy - 4 single bonds and 2 lone pairs. Enthalpy of Formation. Chemical Bonds. Stefan V. Metric Prefixes. Titrations: Weak Acid-Strong Base. Amphoteric Species. Density of Geometric Objects. Lattice Energy. Carboxylic Acid Reactions.
IF4- lewis structure has an Iodine atom I at the center which is surrounded by four Fluorine atoms F.
Question bf. Boiling Point Elevation. Law of Multiple Proportions. Enthalpy of Formation. Back to all problems. Multiplication and Division Operations. Balancing Chemical Equations. Band of Stability: Overview. Atomic Theory. Density of Geometric Objects. Measuring Radioactivity. Wavelength and Frequency. Osmotic Pressure. Parts per Million ppm. Group 1A and 2A Reactions.

I apologise, but, in my opinion, you are not right. I can defend the position. Write to me in PM, we will talk.