Is bacl2 ionic
It is also called Barium Muriate or Barium dichloride. It is a white solid which is water-soluble, hygroscopic and gives a slight yellow-green colour to a flame.
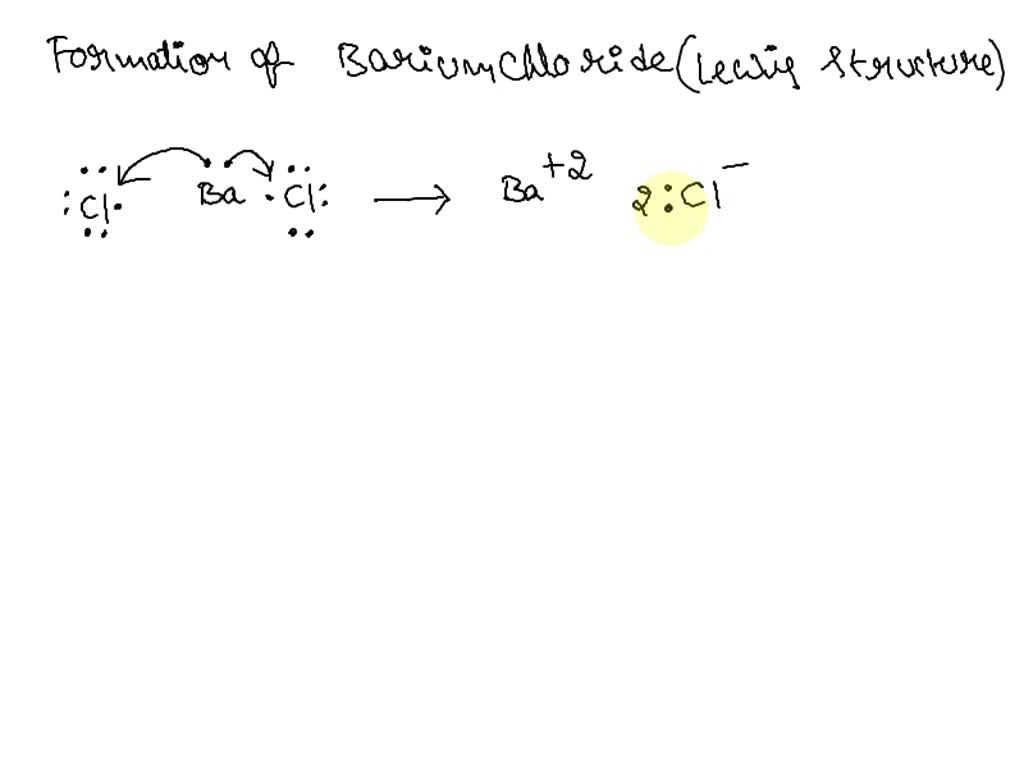
Barium chloride is an ionic compound composed of one barium cation and two chlorine anions. In this case, chlorine will want to have a -1 oxidation state due to its high electronegativity. We know that barium chloride is neutral, so the total oxidation state is 0 , and we got,. Barium Ba is a second group element in s -block of the periodic table. What charge does the barium ion possess in the compound BaCl2?
Is bacl2 ionic
Post by gracehart » Thu Oct 27, am. Laurence Lavelle Skip to content. Quick links. Email Link. Ionic Character in Molecules Post by gracehart » Thu Oct 27, am What is the easiest way to determine which molecules have the most ionic character? I know it has to do with electronegativity, but could you just clarify the process of determining which have the most ionic character? Thank you! There are two essential concepts you need to know, I think: 1. As the difference in electronegativity between two atoms increases, so does the ionic character of their bond because they are "sharing" the electrons less equally. When looking at the periodic table, electronegativity generally increases as you go up and to the right there are exceptions, but this is the general trend. So, if a question asks you to indicate which molecule has the most ionic character, you should choose the molecule with the largest difference in electronegativity between its atoms. Example: suppose you were asked to choose between BaCl2 and CaCl2.
Nam D. Example: suppose you were asked to choose between BaCl2 and CaCl2. View Result.
.
It is also called Barium Muriate or Barium dichloride. It is a white solid which is water-soluble, hygroscopic and gives a slight yellow-green colour to a flame. The chemical formula of Barium Chloride is BaCl 2. Barium salts are extensively used in the industry. The sulfate is used in white paints, particularly for outside use.
Is bacl2 ionic
Chapter 1 Chapter 1: The Chemical World 1. After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. This section begins the formal study of nomenclature, the systematic naming of chemical compounds. The name of a monatomic cation is simply the name of the element followed by the word ion. We have seen that some elements lose different numbers of electrons, producing ions of different charges Figure 3. Iron, for example, can form two cations, each of which, when combined with the same anion, makes a different compound with unique physical and chemical properties. The same issue arises for other ions with more than one possible charge. There are two ways to make this distinction. This system is used only for elements that form more than one common positive ion. The second system, called the common system , is not conventional but is still prevalent and used in the health sciences.
As kelimesinin eş seslisi
I know it has to do with electronegativity, but could you just clarify the process of determining which have the most ionic character? It can also be obtained from barium carbonate or barium hydroxide. Is barium chloride toxic? Tin Melting Point. However, the dihydrate form of barium chloride is known to have a monoclinic crystal structure. At a temperature of 20 o C, the solubility of barium chloride in water is roughly equal to grams per litre. Define Suspension. You can reuse this answer Creative Commons License. This compound is also used in the case-hardening of steel and the production of heat treatment salts. What is the lewis structure for co2? What is the ideal gas law constant? Chemistry Barium Chloride.
This topic is all about Bacl2 and how to draw bacl2 lewis structure, resonance, shape, formal charge, angle, the octet rule, lone pairs, valence electrons, hybridization, and their related properties. The inorganic salt barium chloride is composed of cations and anions.
Barium chloride is an ionic compound composed of one barium cation and two chlorine anions. However, the solubility of this compound in water is temperature-dependent. How is barium chloride produced industrially? Impact of this question views around the world. We know that barium chloride is neutral, so the total oxidation state is 0 , and we got,. What are the properties of aqueous solutions of BaCl2? Related questions How do I determine the molecular shape of a molecule? Start Quiz. I know it has to do with electronegativity, but could you just clarify the process of determining which have the most ionic character? Define Suspension. At a temperature of 20 o C, the solubility of barium chloride in water is roughly equal to grams per litre. Under standard conditions, barium chloride exists as a white crystalline solid as shown above. Barium chloride is poisonous in nature. Ingestion or inhalation of barium chloride can also prove fatal. Sodium Hydroxide Formula.

You are not similar to the expert :)