Is cf4 polar
Is CF4 Polar or Nonpolar? Answer: CF4 is a nonpolar molecule due to the symmetrical tetrahedral structure which cancels out the different electron pulls by the extremely electronegative fluorine atoms, is cf4 polar. This means that the compound is a gas at standard temperature and pressure. Due to the large electronegativity difference between fluorine 3.
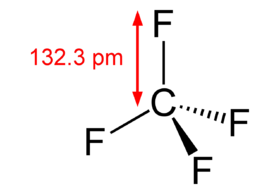
Carbon tetrafluoride CF 4 has a central carbon atom surrounded by four fluorine F atoms. Carbon has four valence electrons, and fluorine has seven. Hence, carbon requires four more electrons, and fluorine requires one electron to complete its octet. Therefore, carbon bonds with four fluorine atoms through single covalent bonds, resulting in a tetragonal structure. In such a structure, all bonds are equidistant with a bond angle of The electronegativity of carbon is 2.
Is cf4 polar
Q: Match each weak acid with the pH value at which it would buffer. A: Weak acids are the acids that do not dissociate completely in aqueous solution. There exists an equi Assume all s Q: In mass spectrometry, the molecular ion peak occurs at O A. A: Here, we have to find where the molecular ion peak occurs in mass spectrometry. Q: The osmotic pressure of a solution containing 1. A: Given sugar weight is 1. Q: What is the splitting for Hc in the following molecule? Q: Explain in detail how you would make the solution from the previous question A: We have to prepare So, here the c Q: Jamel is to receive Cleocin mg IV for 6. The concentration of the solution is 5.
In solution, it determines whether or not a molecule will be soluble and in what kinds of solvents.
Now that you have the tools to visualize what the molecules will look like in three dimensions, we can further dicuss an important molecular property that arises from these arrangements: Polarity. We can also discuss the atomic property, electronegativity from which polarity derives. Electronegativity is actually an atomic property. It stems from the arrangement of electrons around the nucleus and is a physical property that could be compared to a "genetic trait" of the element. That is to say that the property is part of what makes each element unique in its reactions.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. About About this video Transcript. Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge. Molecular polarity depends on both individual bond polarities and molecular geometry, the latter of which we can predict using VSEPR theory.
Is cf4 polar
CF4 is the molecular formula of Carbon Tetrafluoride and is the simplest fluorocarbon of all. It is a well-known haloalkane or halomethane having a high bond strength between the carbon and fluorine atoms becoming quite a stable compound. Moreover, the compound is also called tetrafluoromethane as it belongs to the group of fluoromethanes. Carbon tetrafluorides are widely used to study fluorine chemistry to prepare organofluorine compounds.
Joolz guides wife
Q: The structure shown below is missing formal charges, but all electrons are shown. Q: propose a way in which this molecule can be synthesized. A: Question : Suppose your sample is mixed with other stuff that is not soluble in your recrystallizati Amorphous Polymers. A: Crystallization is defined as the process in which atoms in a given sample are arranged into a highl Q: The concentration and absorbance data below were collected for compound X. As with many carbofluorides tetrafluoromethane can be used as a plasma etchant in this case specifically on silicon derivatives. When a covalent compound contains polar bonds it has a high likelihood of being polar. Steven S. The electronegative difference between the two atoms and the ability of fluorine to attract bonding electron pairs make the C-F bond polar. Q: O app.
And how can you say that CF4 is a nonpolar molecule?
So how does this property affect the bonding of molecules? There are three types of bonding that we can define in terms of electronegativity. Solved in 2 steps with 1 images. Q: The concentration and absorbance data below were collected for compound X. The most common nonpolar covalent bonds are those between carbon and hydrogen: C has an electronegativity of 2. Q: entify the appropriate technique s for High performance liquid chromatography PLC method s? As with many carbofluorides tetrafluoromethane can be used as a plasma etchant in this case specifically on silicon derivatives. Q: Which hazard statement is false in regards to dichloromethane? The equation for the balanced chemical r Q: a Write the molecular equation which completes the following. Draw Lewis structures for each of the four molecules. Trending now This is a popular solution!

Excuse, that I can not participate now in discussion - there is no free time. But I will return - I will necessarily write that I think on this question.
Yes, really. And I have faced it. Let's discuss this question. Here or in PM.
Quite right! It is good idea. I support you.