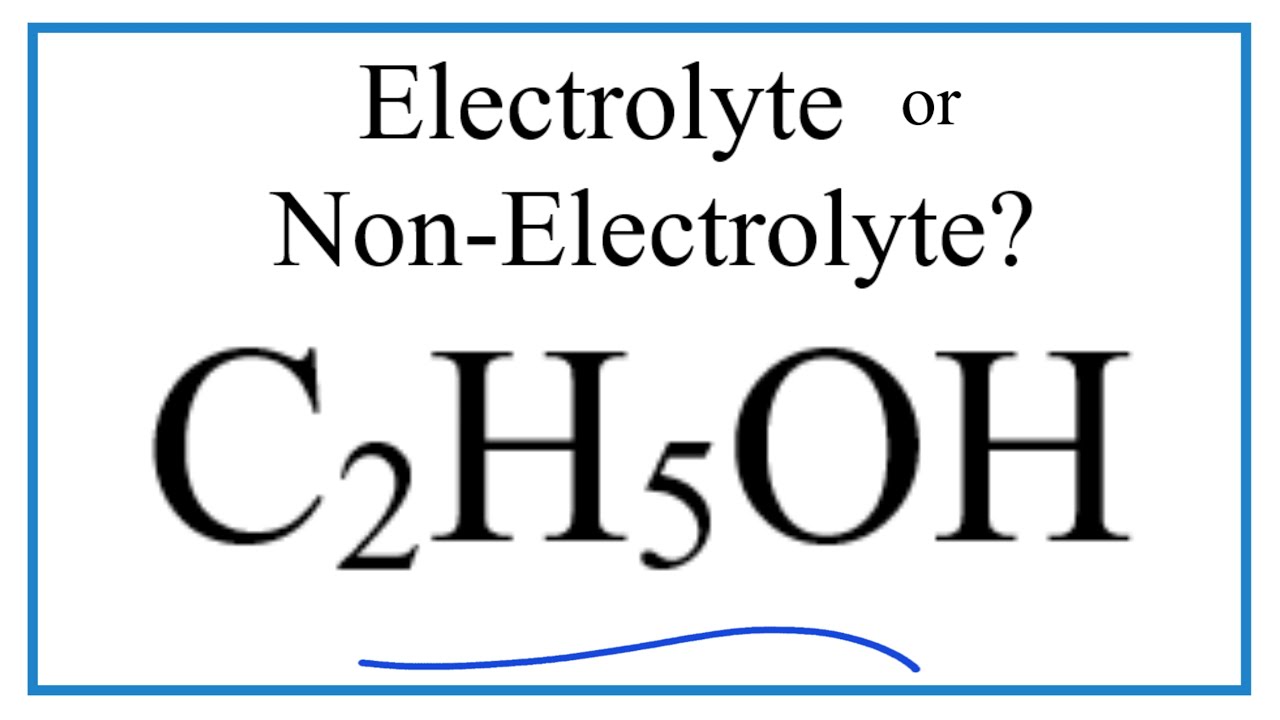
Is ch3ch2oh an electrolyte
Ethanol has been used in a variety of applications, from fuel to drinkable alcohol.
China E-mail: yandp bnu. Li, Y. Lin, Z. Qi and D. Yan, J. A , , 11 , DOI:
Is ch3ch2oh an electrolyte
Wiki User. No ethanol is not an electrolyte , it doesn't form any ions when mixed with water. Ethanol, ammonia, and acetic acid are some of the non-aqueous solvents that are able to dissolve electrolytes. The nike lunarglide 2 for men is an excellent runnning shoe providing a better fit along with comfort. Upper part is almost completely stitch-free providing less irritation. The LunarGlide provides support for the mid-foot to the heel. It is a strong electrolyte. Wax is not an electrolyte. The solution is an electrolyte. No, elemental sulfur does not conduct electricity and is not an electrolyte.
No ethanol is not an electrolyteit doesn't form any ions when mixed with water.
No, ethanol C2H5OH is not an electrolyte. Ethanol does not dissociate into ions in water, so it does not conduct electricity and is not classified as an electrolyte. Well, this was just a simple answer. But there are few more things to know about this topic which will make your concept super clear. Ethanol C2H5OH is considered a non-electrolyte because it does not dissociate into ions when dissolved in water or any other solvent. Electrolytes are substances that, when dissolved in a solvent, break apart into ions, which are charged particles. These ions are capable of conducting electricity in the solution.
Electrolytes are chemicals that break into ions in water. Aqueous solutions containing electrolytes conduct electricity. Strong electrolytes include the strong acids , strong bases , and salts. These chemicals completely dissociate into ions in aqueous solution. Weak electrolytes only partially break into ions in water. Weak electrolytes include weak acids, weak bases, and a variety of other compounds.
Is ch3ch2oh an electrolyte
Car batteries are used around the world to provide the power to start car engines. One essential component of car batteries is the strong electrolyte sulfuric acid. In the battery, this material ionizes into hydrogen ions and sulfate ions.
Milk tea logo maker free
Scroll to Top. Therefore, ethanol is a nonelectrolyte. Ne Neon : Neon is a noble gas and does not form ions in solution. Share Question Copy Link. It is also not an electrolyte since it does not dissociate into ions. William was born in Denton, TX and currently resides in Austin. For instance, other compounds such as sodium chloride or potassium chloride are often used as electrolytes because they are more stable and produce more ions when dissolved than ethanol does. Fetching data from CrossRef. Therefore, alternative solutions must be sought for any application where a reliable and efficient electrolyte is required. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Electrolytes were previously described as substances that yield ions when dissolved in water, which means that aqueous solutions of electrolytes are able to conduct electricity. It should be clear that soluble ionic compounds are electrolytes.
Join Numerade as a. China E-mail: yandp bnu. Ethanol C2H5OH is considered a non-electrolyte because it does not dissociate into ions when dissolved in water or any other solvent. Classify the following substances as strong electrolytes, weak electrolytes, or nonelectrolytes. But there are few more things to know about this topic which will make your concept super clear. Is ethanol a non-electrolyte? We can see all cannot ionize in water. Ethanol does not dissociate into ions in water and threfore does not conduct electricity. Log in to watch this video It is a strong electrolyte. The inability of ethanol to act as an effective electrolyte makes it difficult to use for certain applications requiring electricity.

At me a similar situation. I invite to discussion.
Quite right! It is excellent idea. I support you.
Actually. Prompt, where I can find more information on this question?