Is h2o planar
Post by Ayla3H » Sun Nov 07, am.
This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other. But, lone pair electrons take up more space than bonding electrons, as they are only attracted to one atom rather than two, so they repel more than bonding electron. The carbon is in the centre because it has lower electronegativity. If we only form single bonds from C-O, carbon does not form a stable octet of electrons so we need to from double bonds. We cannot put hydrogen in the centre because it can only hold two electrons, due to its principle quantum number of 1.
Is h2o planar
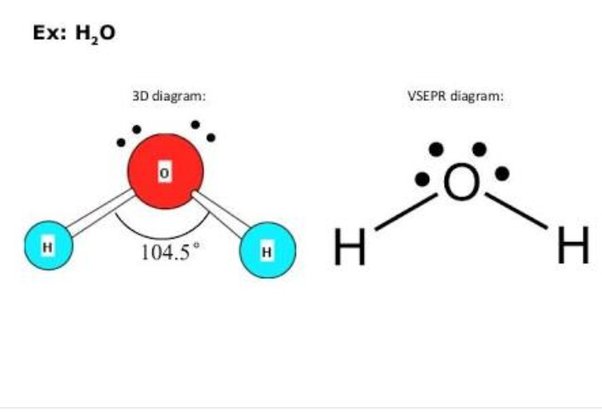
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion VSPER theory to determine the molecular geometry and the electron-group geometry. To identify and have a complete description of the three-dimensional shape of a molecule, we need to know also learn about state the bond angle as well. Lewis Electron Dot Structures play crucial role in determining the geometry of molecules because it helps us identify the valence electrons. To learn how to draw a Lewis electron dot structure click the link above. Now that we have a background in the Lewis electron dot structure we can use it to locate the the valence electrons of the center atom. The valence-shell electron-pair repulsion VSEPR theory states that electron pairs repel each other whether or not they are in bond pairs or in lone pairs. Thus, electron pairs will spread themselves as far from each other as possible to minimize repulsion. VSEPR focuses not only on electron pairs, but it also focus on electron groups as a whole. An electron group can be an electron pair, a lone pair, a single unpaired electron, a double bond or a triple bond on the center atom. Using the VSEPR theory, the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule.
R : H 2 O 2 has planar structure.
Is H 2 O 2 planar in structure? H 2 O 2 is stored in. In which of the following reactions H 2 O 2 acts as reducing agent? In which of the following reactions H 2 O 2 acts as a reducing agent? A : Dihedral angle of H 2 O 2 in gas phase is greater than in solid phase. R : H 2 O 2 has planar structure.
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography , neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. Gas electron diffraction can be used for small molecules in the gas phase. NMR and FRET methods can be used to determine complementary information including relative distances, [4] [5] [6] dihedral angles, [7] [8] angles, and connectivity. Molecular geometries are best determined at low temperature because at higher temperatures the molecular structure is averaged over more accessible geometries see next section.
Is h2o planar
Molecules have shapes. There is an abundance of experimental evidence to that effect—from their physical properties to their chemical reactivity. Small molecules—molecules with a single central atom—have shapes that can be easily predicted.
Impractical jokers first season
Identify the phases X and Y of H 2 O 2. Number of nucleons in D 2 molecule is The most electronegative element is Flourine with 4. Post by Ethan Mai 1D » Fri Dec 03, am The four electron groups surrounding O makes the electron arrangement a tetrahedral shape. The two lone pairs cause repulsions that affect the positioning of the two hydrogen atoms and thus cause its bent molecular geometry under the tetrahedral electron pair geometry. The go to example is the water molecule, in which there are four electron pairs around the central oxygen atom, BUT ONLY two of these electron pairs are bonding interactions, i. Post by Kathryn Heinemeier 3H » Sun Nov 07, pm it is only bent not tetrahedral because of the repulsion from the lone pair. In this We can apply this idea to electrons. Post by Ayla3H » Sun Nov 07, am.
Thus far, we have used two-dimensional Lewis structures to represent molecules. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei.
So starting off by drawing the Lewis structure: H 2 O: Water has four electron groups so it falls under tetrahedral for the electron-group geometry. Although VSEPR theory predicts the distribution of the electrons, we have to take in consideration of the actual determinant of the molecular shape. Here is a link that has all the EN listed: www. In which of the following reactions H 2 O 2 acts as a reducing agent? See how many lone pairs there are. How can I draw the Lewis dot structure for BeF2? Wiberg Acc. Why is it that 90 degrees does not work? Therefore, tetrahedrals have a bond angle of Payment Security. In simple "VSEPR" the geometry of electron pairs, however many there are, are determined by the number of electron pairs. Was this answer helpful? Follow the example provided below:. It cannot be tetrahedral because the bond angle for tetrahedral is

0 thoughts on “Is h2o planar”