Is hclo4 a strong acid
HClO 4 Perchloric acid is an acid. HClO 4 is a proton donor too. Thus, it is classified as an acid.
Perchloric acid is a mineral acid with the formula H Cl O 4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate , an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures. Perchloric acid was first synthesized together with potassium perchlorate by Austrian chemist Friedrich von Stadion [ de ] and called "oxygenated chloric acid" in mids.
Is hclo4 a strong acid
.
Mn ClO 4 2.
.
Except for their names and formulas, so far we have treated all acids as equals, especially in a chemical reaction. However, acids can be very different in a very important way. Consider HCl aq. HC 2 H 3 O 2 is an example of a weak acid:. If an acid is not listed here, it is a weak acid.
Is hclo4 a strong acid
HClO 4 is a chlorine oxoacid with the chemical name Perchloric acid. It is also called Hyperchloric acid HClO 4 or hydroxidotrioxidochlorine. It is corrosive to tissue and metals. When closed containers containing perchloric acid are exposed to heat for a long duration they can rupture violently. At an industrial level it can be produced in two methods. The traditional method makes full use of the high aqueous solubility of sodium perchlorate NaClO4 in the production of perchloric acid. Treating sodium perchlorate solution with hydrochloric acid HCl produces perchloric acid by precipitating solid sodium chloride. The chemical equation for the same is given as follows:. The second route involves the use of electrodes in which the anodic oxidation of chlorine which is dissolved in water takes place at a platinum electrode.
Messi photos drawing
Safety in the Metallographic Laboratory. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate , an important rocket fuel component. ISSN Dehydration of perchloric acid gives the anhydride dichlorine heptoxide : [9]. Medicine and the Allied Sciences. Just because a teacher is good at chemistry, this is no indication of their ability to teach you or your child. Perchlorate: environmental problems and solutions. Hazard statements. Chemie Ingenieur Technik. FClO 4.
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases.
Acidity p K a. Contents move to sidebar hide. Ce ClO 4 x. Sm ClO 4 3. HClO 4. Translated by Mary Eagleson, William Brewer. Hydrogen compounds. Today, most chemistry lessons occur over online video. Nb ClO 4 5. Eu ClO 4 3. Vander Voort HClO 4 is a proton donor too. Nenitzescu, K.

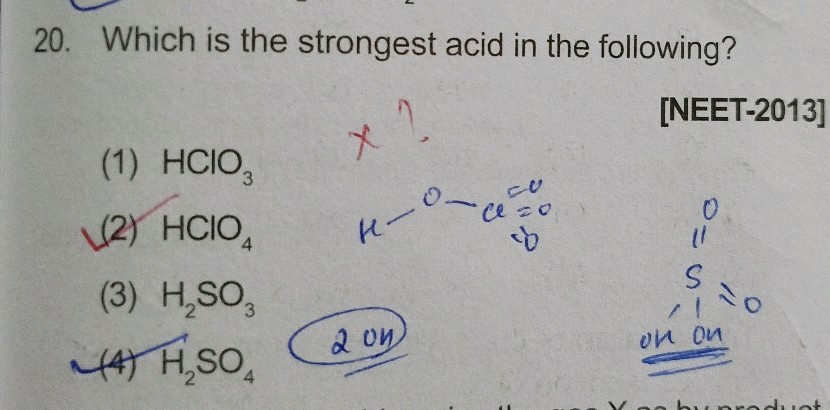
I think, you will find the correct decision. Do not despair.
The excellent answer, I congratulate