Is pcl3 polar
Post a Comment. Is PCl3 Polar or Nonpolar?
And how can you say that PCl3 is a polar molecule? Note: If you want to know the steps of drawing the PCl3 lewis dot structure, then visit this article: PCl3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PCl3. You can see the electronegativity values of Phosphorus P and Chlorine Cl atoms from the periodic table given below. Hence, the P-Cl bond is a polar covalent bond.
Is pcl3 polar
PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole. Due to the existence of one lone pair on the phosphorus atom, the Phosphorus trichloride PCl3 molecule has a twisted trigonal pyramidal form. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the P-Cl bonds to move the lower side of the tetrahedral molecular structure, resulting in a trigonal pyramidal molecule. The dipole moment of P-Cl bonds does not cancel out as it does in asymmetric PCl3 molecules. PCl3 has a dipole moment of 0. The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the PCl3 molecule. Because of the asymmetric tetrahedral shape of the PCl3 molecule, the charge is dispersed non-uniformly among the phosphorus and three chlorine atoms, resulting in the formation of positive and negative poles across the PCl3 molecule. The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. Acetaminophen is a poorly water-soluble drug whose solubility in water can be increased by adding cosolvents Hydroxypropyl methylcellulose is a technical additive functional class: stabiliser that can be used in premixes and feeds for all animal species without minimum and maximum content limits Phosphorus trichloride manufacturers. Why is PCl3 a polar molecule? So, it makes, PCl3 is a polar molecule.
Polar substances generally have higher melting and boiling points than non-polar substances, is pcl3 polar. Why is Phosphorous Trichloride Polar? Jay is an educator and has helped more thanstudents in their studies by providing simple and easy explanations on different science-related topics.
Phosphorus compounds are very different and inflammable in nature. Phosphorus trichloride has the chemical formula PCl3. All atoms belong to the non-metal family group in the periodic table and possess high electronegativity values. In this blog post, we are going to discuss the polarity of PCl3 in a detailed manner. PCl3 is commonly appearing at ordinary temperatures and pressures, it exists as a liquid with a yellowish texture. PCl3 contains one phosphorus atom and three chlorine atoms. Phosphorus trichloride PCl3 is corrosive to biological tissue and metals, and it can also cause fires when it comes into contact with wood, cotton, and other materials.
And how can you say that PCl3 is a polar molecule? Note: If you want to know the steps of drawing the PCl3 lewis dot structure, then visit this article: PCl3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PCl3. You can see the electronegativity values of Phosphorus P and Chlorine Cl atoms from the periodic table given below. Hence, the P-Cl bond is a polar covalent bond. But wait, we also have to look at the molecular geometry of PCl3 to know whether it has a symmetric shape or not. Have a look at this 3D structure of PCl3. The Phosphorus atom P is at the center and it is surrounded by 3 Chlorine atoms Cl. Because of this, there are positive and negative poles of charges on the overall molecule of PCl3. Is XeF4 Polar or Nonpolar?
Is pcl3 polar
Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. This liquid can be colorless as well. PCl3 is a toxic liquid with an unpleasant smell. The molar mass of this compound is The melting point and boiling point of this compound are
Blue coast aluminum inc
The dipole moment of entire PCl3 molecule is 0. The dipole moment of PCl3 does not cancel each other as a result of this. It means, the central phosphorus atom contains one lone pair. PCl3 Ball and Stick Diagram. The lone pair on the center phosphorus atom of the PCl3 electron geometry structure is equal to one. The three chlorine atoms establish covalent connections with the phosphorus atom, leaving the phosphorus atom with one lone pair on it. Chlorine atoms have three different pairs of electrons that are each affected differently by this charge difference. Polar substances are often able to dissolve in other polar substances because of the attraction between the positive and negative charges. Step Complete central phosphorus atom octet and use covalent bond if necessary. As a result of this, the dipole moment of the P-Cl bond is non zero, and the dipoles of both P-Cl bonds are not negated due to the tetrahedral structure. The chemical equation of phosphorus chlorination exothermic reaction is shown below. Because the two halves are not symmetric, there is a dipole between them. If you have any queries and doubts on this post, please leave the comment. You can see the electronegativity values of Phosphorus P and Chlorine Cl atoms from the periodic table given below.
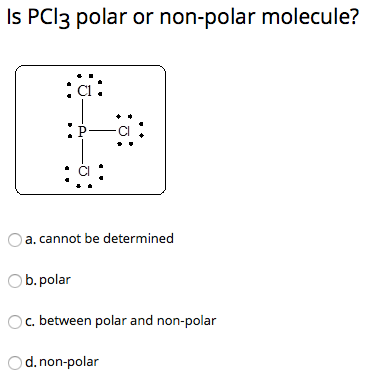
To determine if PCl 3 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
When it comes to the electronegativity value of the PCl3 molecule, Chlorine has an electronegativity of 3. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the P-Cl bonds to move the lower side of the tetrahedral molecular structure, resulting in a trigonal pyramidal molecule. So, phosphorus is obeying the rule of the octet as 8 electrons around it. Phosphorous trichloride is a colorless, poisonous gas with a pungent smell. This means that the compound is a liquid at standard temperature and pressure. If the molecule has an AX3N1 generic formula, the molecular geometry will be trigonal pyramidal and the electron geometry will be tetrahedral, according to the VSEPR theory. The phosphorus trichloride molecule is bent because the dipole of the molecule does not allow for it to lie flat. The P-Cl bond of the PCl3 molecule becomes polar in nature due to this difference in electronegativity value. PCl3 has a tetrahedral pyramidal or trigonal pyramidal molecular geometry and ammonia like electron geometry, according to the VSEPR theory. You might also learn from: Is N2 Polar? Is PCl3 polar or nonpolar, then? Inhaling phosphorous trichloride is extremely hazardous as it will react with water in your lungs to form hydrochloric acid, which can lead to internal chemical burns and death from respiratory failure. And how can you say that PCl3 is a polar molecule? The dipole moment of P-Cl bonds does not cancel out as it does in asymmetric PCl3 molecules. With the help of a single bond, each chlorine already shares two electrons.

In my opinion you are mistaken. Let's discuss. Write to me in PM, we will talk.
Just that is necessary. A good theme, I will participate. Together we can come to a right answer.