Is sf4 a polar molecule
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of is sf4 a polar molecule pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar!
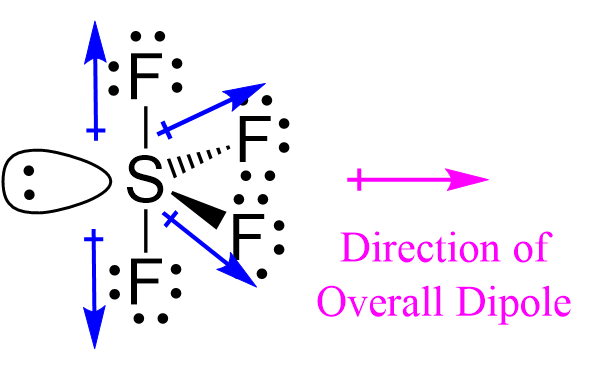
To determine if SF 4 i spolar or not, we need to first draw its Lewis structure and determine the geometry. We came up with the following Lewis structure in the previous post , so feel free to check it:. The central atom has 4 atoms connected to it, and one lone pair, therefore, the electron geometry is trigonal bipyramidal while the molecular geometry is seesaw :. Now, the polarity: The first thing here is to determine if the S-F bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. The difference in electronegativities is 1.
Is sf4 a polar molecule
Non-polar compounds are soluble in non -polar solvents. What is a polar and non-polar molecule? What are polar and non-polar molecules? Molecule having non-polar as well as polar bonds but the molecule as a whole is polar. Why C O 2 is non-polar but S O 2 is polar? Distinguish between polar and non-polar molecules. What are polar and non-polar covalent bonds? The interaction between a polar and non-polar moleclues known as. Give their examples. Which is non -polar ,but contains polar bonds? Distinguish between polar molecules and non-polar molecules. B F 3 is non-polar but N H 3 is polar. The motion of a rocket is based on the principle of conservation ofa l Ideal solution Positive deviation Negative deviation.
Open in App. Preparation of Aluminium Chloride. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids.
Post by Angeline 3E » Mon Nov 18, am. Laurence Lavelle Skip to content. Quick links. Email Link. Why is SF4 Polar? Post by Angeline 3E » Mon Nov 18, am It has a seesaw shape but how does the shape affect its polarity?
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and type of atoms present in the given compound. Here there is one sulfur atom and four fluorine atoms in the compound, which makes it similar to the molecular formula of AX4E. Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. As there is one lone pair on the central atom, it repels the bonding pair of electrons, which tweaks the shape a little bit and makes it appear like a see-saw.
Is sf4 a polar molecule
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar!
Ninja talent
You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. Sulphur Tetrafluoride contains 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the core atom in its Lewis structure. Since the seesaw shape is not symmetrical, the S-F bond energies do not cancel and there is polarity. Steps in the Ring Closure. Why are polar molecules hydrophilic? Chemistry Intermolecular Bonding Polarity of Molecules. Jump to. Ans : S — atom in SF Post by Robert Tran 1B » Mon Nov 18, pm Since the S--F bond has a dipole moment and the seesaw shape does not allow the dipole moments to cancel out, the molecule is polar. Their bond dipoles do not cancel, so the molecule is polar. How can I calculate the polarity of a solvent? In order to minimize the repulsive forces between them, electron pairs around the central atom, tend to stay as far away from each other as possible. Electron pairs around the molecule's central atom can be shared or can be lone pairs. Covalent and Ionic Bonds.
Sulfur tetrafluoride is a chemical compound with its chemical formula SF4. This compound exists as a colorless gas.
Email Link. Oxalic-Acid vs KMnO4. Open in App. Covalent and Ionic Bonds. Who is online Users browsing this forum: No registered users and 8 guests. Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bo Post by Angeline 3E » Mon Nov 18, am. Two of the S-F bonds are pointing away from each other, and their bond dipoles cancel. How does polarity relate to electronegativity? In order to minimize the repulsive forces between them, electron pairs around the central atom, tend to stay as far away from each other as possible. Enthalpy of Neutralisation. The interaction between a polar and non-polar moleclues known as.

0 thoughts on “Is sf4 a polar molecule”