J.j thomson facts
Sir Joseph John Thomson, often known as J. His father, a bookseller, wanted him to be an engineer, but did not have the fee for J.
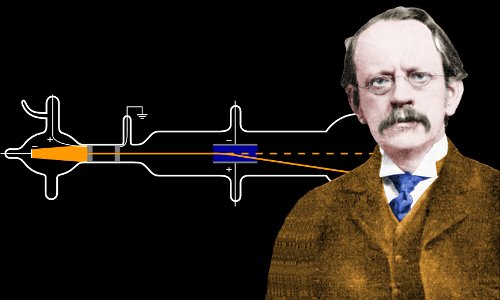
In , Thomson showed that cathode rays were composed of previously unknown negatively charged particles now called electrons , which he calculated must have bodies much smaller than atoms and a very large charge-to-mass ratio. His experiments to determine the nature of positively charged particles, with Francis William Aston , were the first use of mass spectrometry and led to the development of the mass spectrograph. Thomson was awarded the Nobel Prize in Physics for his work on the conduction of electricity in gases. His mother, Emma Swindells, came from a local textile family. His father, Joseph James Thomson, ran an antiquarian bookshop founded by Thomson's great-grandfather. He had a brother, Frederick Vernon Thomson, who was two years younger than he was.
J.j thomson facts
In Thomson discovered the electron and then went on to propose a model for the structure of the atom. His work also led to the invention of the mass spectrograph. Later he estimated the value of the charge itself. In Thomson suggested a model of the atom as a sphere of positive matter in which electrons are positioned by electrostatic forces. His efforts to estimate the number of electrons in an atom from measurements of the scattering of light, X, beta, and gamma rays initiated the research trajectory along which his student Ernest Rutherford moved. Here his techniques led to the development of the mass spectrograph. Ironically, Thomson—great scientist and physics mentor—became a physicist by default. His father intended him to be an engineer, which in those days required an apprenticeship, but his family could not raise the necessary fee. Instead young Thomson attended Owens College, Manchester, which had an excellent science faculty. He was then recommended to Trinity College, Cambridge, where he became a mathematical physicist. In he was named to the prestigious Cavendish Professorship of Experimental Physics at Cambridge, although he had personally done very little experimental work. Even though he was clumsy with his hands, he had a genius for designing apparatus and diagnosing its problems. He was a good lecturer, encouraged his students, and devoted considerable attention to the wider problems of science teaching at university and secondary levels. Of all the physicists associated with determining the structure of the atom, Thomson remained most closely aligned to the chemical community. His nonmathematical atomic theory—unlike early quantum theory—could also be used to account for chemical bonding and molecular structure see Gilbert Newton Lewis and Irving Langmuir.
His professor of mathematics recognized his brilliance, and he was encouraged to apply for a scholarship at Trinity College in Cambridge.
Sir Joseph John Thomson 18 December — 30 August was an English physicist and Nobel Laureate in Physics , credited with the discovery and identification of the electron ; and with the discovery of the first subatomic particle. In , Thomson showed that cathode rays were composed of previously unknown negatively charged particles, which he calculated must have bodies much smaller than atoms and a very large value for their charge-to-mass ratio. Thomson is also credited with finding the first evidence for isotopes of a stable non-radioactive element in , as part of his exploration into the composition of canal rays positive ions. His experiments to determine the nature of positively charged particles, with Francis William Aston , were the first use of mass spectrometry and led to the development of the mass spectrograph. Thomson was awarded the Nobel Prize in Physics for his work on the conduction of electricity in gases.
In Thomson discovered the electron and then went on to propose a model for the structure of the atom. His work also led to the invention of the mass spectrograph. Later he estimated the value of the charge itself. In Thomson suggested a model of the atom as a sphere of positive matter in which electrons are positioned by electrostatic forces. His efforts to estimate the number of electrons in an atom from measurements of the scattering of light, X, beta, and gamma rays initiated the research trajectory along which his student Ernest Rutherford moved.
J.j thomson facts
Sir Joseph John Thomson or J. Thomson is best known as the man who discovered the electron. He died August 30, , Cambridge, Cambridgeshire, England. Thomson is credited with the discovery of the electron , the negatively charged particle in the atom. He is known for the Thomson atomic theory.
Ann clark cookie cutters canada
Writers Directory. Several scientists, such as William Prout and Norman Lockyer , had suggested that atoms were built up from a more fundamental unit, but they envisioned this unit to be the size of the smallest atom, hydrogen. He was then recommended to Trinity College, Cambridge, where he became a mathematical physicist. International Mass Spectrometry Foundation. By comparing the deflection of a beam of cathode rays by electric and magnetic fields he obtained more robust measurements of the mass-to-charge ratio that confirmed his previous estimates. In , Thomson demonstrated that hydrogen had only a single electron per atom. He was a good lecturer, encouraged his students, and devoted considerable attention to the wider problems of science teaching at university and secondary levels. Influenced by the work of James Clerk Maxwell land the discovery of the X-ray, Thomson deduced that cathode rays produced by Crookes tube exhibited a single charge-to-mass ratio e m and must be composed of a single type of negatively charged particle, which he called "corpuscles. List of scientists whose names are used as SI units and non SI units. Rose attended demonstrations and lectures, among them Thomson's, leading to their relationship. Thomson is the father of Nobel laureate George Paget Thomson. Scientists whose names are used in physical constants. Thomson made his suggestion on 30 April following his discovery that cathode rays at the time known as Lenard rays could travel much further through air than expected for an atom-sized particle.
December 18 , Died On : August 30 ,
In , Thomson demonstrated that hydrogen had only a single electron per atom. The Timetables of Science. Thomson published a number of papers addressing both mathematical and experimental issues of electromagnetism. All content from Kiddle encyclopedia articles including the article images and facts can be freely used under Attribution-ShareAlike license, unless stated otherwise. Thomson himself remained critical of what his work established, in his Nobel Prize acceptance speech referring to "corpuscles" rather than "electrons". When the upper plate was connected to the negative pole of the battery and the lower plate to the positive pole, the glowing patch moved downwards, and when the polarity was reversed, the patch moved upwards. Millikan 's oil drop experiment in His experiments to determine the nature of positively charged particles, with Francis William Aston , were the first use of mass spectrometry and led to the development of the mass spectrograph. Previous theories allowed various numbers of electrons. Laureates of the Nobel Prize in Physics. One of his students was Ernest Rutherford, who would later succeed him in the post. Wilson O.

0 thoughts on “J.j thomson facts”