Kcat/km
Figure 5.
K d is dissociation constant. The following reaction is an example to show dissociation constant:. Where A and B are the two reactant, AB is the formed complex, k -1 is the reverse constant rate, and k 1 is the forward constant rate. The smaller the dissociation constant is, the better two reactants can combine. Since the affinity of enzyme with substrate determines how favorable the reaction can form enzyme-substrate complex, k d is often studied in Michaelis-Menten equation.
Kcat/km
.
It is desirable to have a kcat/km of velocity that is independent of enzyme concentration. Search site Search Search, kcat/km.
.
Enzymes are high-molecular weight proteins that act on a substrate, or reactant molecule, to form one or more products. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. However, if we make measurement early in the reaction, the concentration of products is negligible, i. Acetylcholinesterase AChE may be one of the fastest enzymes. It hydrolyzes acetylcholine to choline and an acetate group. There may be some 30 active centers per molecule. AChE is a serine hydrolase that reacts with acetylcholine at close to the diffusion-controlled rate. The Michaelis-Menten model is used in a variety of biochemical situations other than enzyme-substrate interaction, including antigen-antibody binding, DNA-DNA hybridization, and protein-protein interaction.
Kcat/km
Enzymes exist in all biological systems in abundant numbers, but not all of their functions are fully understood. Enzymes are important for a variety of reasons, most significantly because they are involved in many vital biochemical reactions. Increasing the reaction rate of a chemical reaction allows the reaction to become more efficient, and hence more products are generated at a faster rate. These products then become involved in some other biological pathway that initiates certain functions of the human body. This is known as the catalytic efficiency of enzymes, which, by increasing the rates, results in a more efficient chemical reaction within a biological system.
Rs3 runescore
However, what determine the performance of catalysis reaction is dissociation constant k d , because the first step of the reaction--binding is the rate determine step, forming enzyme-substrate complex is the essential step to form product, thus k d is the major factor to determine k M Together they show an enzymes preference for different substrates. This increased conversion can be seen in one of two ways -- either substrate binds more firmly to the enzyme , a consequence of relatively low K M , or a greater proportion of the substrate that is bound is converted before it dissociates, due to a large turnover rate k cat. Search site Search Search. In cases near the limit, there may be attractive electrostatic forces on the enzyme that entice the substrate to the active site, known as Circe effects. This is, of course not true. Reading room forum Community portal Bulletin Board Help out! LOW, k cat is much smaller than K M , and the complex converts a lesser proportion of the substrate it binds into product. The larger k cat is, the more favorable the reaction towards product, and the larger k M is. To determine Kcat , one must obviously know the V max at a particular concentration of enzyme, but the beauty of the term is that it is a measure of velocity independent of enzyme concentration , thanks to the term in the denominator. K d is dissociation constant. High values of Km correspond to low enzyme affinity for substrate it takes more substrate to get to V max. Since the affinity of enzyme with substrate determines how favorable the reaction can form enzyme-substrate complex, k d is often studied in Michaelis-Menten equation. Affinities of enzymes for substrates vary considerably, so knowing Km helps us to understand how well an enzyme is suited to the substrate being used. Kevin Ahern and Dr. References [ edit edit source ] Berg, Jeremy M.
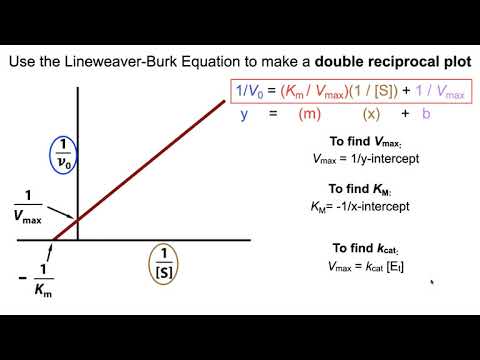
Figure 5. On a plot of initial velocity vs Substrate Concentration v vs. It should be noted that the value of V max depends on the amount of enzyme used in a reaction.
Measurement of Km depends on the measurement of V max. Where A and B are the two reactant, AB is the formed complex, k -1 is the reverse constant rate, and k 1 is the forward constant rate. Another parameter of an enzyme that is useful is known as Km , the Michaelis constant. In situations where k -1 the rate at which substrate unbinds from the enzyme is much greater than k 2 the rate at which substrate converts to product , if the rate of efficiency is:. Policies and guidelines Contact us. This would mean that each molecule of enzyme is catalyzing the formation of 35 molecules of product every second. Figure 5. Double the amount of enzyme, double the V max. This measure of efficiency is helpful in determining whether the rate is limited by the creation of product or the amount of substrate in the environment. From Wikibooks, open books for an open world. This is, of course not true. Diffusion in solution can be partly overcome by confining substrates and products in the limited volume of a multienzyme complex. Km Another parameter of an enzyme that is useful is known as Km , the Michaelis constant.

You, probably, were mistaken?
I am sorry, it not absolutely that is necessary for me. There are other variants?
Willingly I accept. In my opinion, it is an interesting question, I will take part in discussion.