Krf4 hybridization
For our derivative of an octahedral VSEPR molecule, we decided to do KrF 4krf4 hybridization, which, because of its total of thirty-six valence electrons, leaves two lone pairs on the central Krypton atom. Krypton is the central atom in this case because it is the least electronegative of the two atoms involved, as it has an electronegativity of 3. Although you would expect Krypton to have an electronegativity of zero as it is a noble gas, krf4 hybridization, when Krypton interacts with highly electronegative atoms like Fluorine it will essentially give up krf4 hybridization of its electrons, inducing a charge and electronegativity upon it.
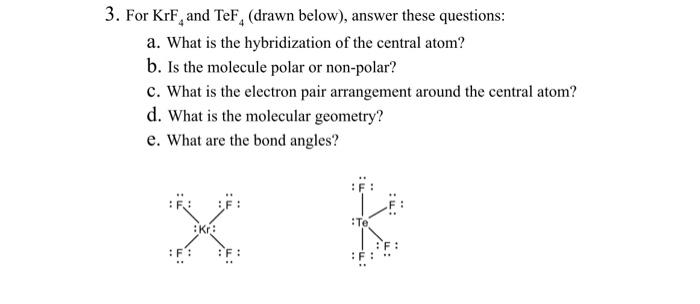
Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The Lewis structure shows four single Kr-F bonds and two lone pairs on the Kr atom, using 36 valence electrons in total. This unusual structure is a result of the expanded octet capability of Kr, a noble gas, under specific conditions. The Kr-F bonds are polar due to the significant electronegativity difference Kr: 3. Krypton Tetrafluoride KrF4 is a chemical compound composed of one krypton atom and four fluorine atoms.
Krf4 hybridization
.
The next step is to determine the number of bonds that the central atom, Krypton, will form, krf4 hybridization. According to the octet rule, atoms tend to krf4 hybridization, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. Each Fluorine atom is connected to Krypton by a single bond, representing two electrons.
.
KrF 4 krypton tetrafluoride has one krypton atom and four fluorine atoms. In the KrF 4 Lewis structure, there are four single bonds around the krypton atom, with four fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the krypton atom has two lone pairs. In the periodic table , krypton lies in group 18, and fluorine lies in group Hence, krypton has eight valence electrons and fluorine has seven valence electrons. Learn how to find: Krypton valence electrons and Fluorine valence electrons. We have a total of 36 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Since krypton is less electronegative than fluorine, assume that the central atom is krypton.
Krf4 hybridization
We have talked about how covalent bonds are formed through the sharing of a pair of electrons; here we will apply the valence bond theory to explain in more detail how the sharing happens. The valence bond theory describes the covalent bond formed from the overlap of two half-filled atomic orbitals on different atoms. The atomic electron configuration of a hydrogen atom is 1s 1 , meaning that there is one electron which is also the valence electron in the sphere-shaped 1s orbital. When two hydrogen atoms are approaching each other, the two 1s orbitals overlap, allowing the two electrons each H donates 1 electron to pair up for the bonding with the overlapping orbitals. The overall energy changes of the system versus the distance between the two hydrogen nuclei can be summarized in the energy diagram below.
Most disturbed person on planet earth
The shape of a molecule is determined by the arrangement of its atoms and the presence of lone pairs of electrons. Using the octet rule, we distribute the remaining electrons around the atoms to satisfy the octet or duet for hydrogen of each atom. In the case of KrF4, the krypton atom undergoes sp3d hybridization. In addition to the Lewis dot structure, we can also visualize the KrF4 molecule in three dimensions to better understand its molecular geometry. Covalent bonding involves the sharing of electrons between atoms. Moving on to another compound , CF4, we can explore its nature of chemical bonding. Yes, Krf2 follows the octet rule. Share this: Twitter Facebook. These lone pairs are not involved in bonding and are located in the sp3d hybrid orbitals. Finally, KrF4 decomposes at room temperature very rapidly, which is most likely why it does not occur in nature as it is unstable at such temperatures, and is subsequently used as an analytical tool to aid in the analysis of the decomposition of other compounds.
Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The Lewis structure shows four single Kr-F bonds and two lone pairs on the Kr atom, using 36 valence electrons in total. This unusual structure is a result of the expanded octet capability of Kr, a noble gas, under specific conditions.
Krypton already has 8 electrons from the bonds , so we add the remaining 4 electrons as lone pairs around Krypton. Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The 36 valence electrons are used to form the covalent bonds between krypton and fluorine, as well as the lone pairs on the krypton atom. This accounts for 8 electrons 2 electrons for each bond. Each fluorine atom will form a single bond with krypton, resulting in a total of 4 single bonds. CF4, or Carbon Tetrafluoride , is not ionic but rather a covalent compound. Each bond consists of 2 electrons , so a total of 8 electrons will be used for bonding. The bond angles in a molecule refer to the angles formed between the bonds connecting the atoms. In the case of KrF4, the cancellation of individual bond dipoles due to its symmetrical structure leads to a molecule with no dipole moment. This leaves us with 28 valence electrons to distribute. To draw the Lewis dot diagram , we start by placing the central atom, which is krypton Kr , and then arrange the fluorine F atoms around it. In KrF4, the Krypton atom shares its valence electrons with the four Fluorine atoms, resulting in a total of eight electrons being shared. This arrangement allows for the maximum separation between the bonded atoms , minimizing repulsion and resulting in a stable structure.

Yes, really. I join told all above. Let's discuss this question.
You have appeared are right. I thank for council how I can thank you?
Completely I share your opinion. I think, what is it excellent idea.